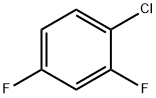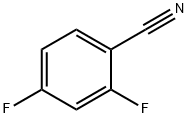
2,4-Difluorobenzonitrile synthesis
- Product Name:2,4-Difluorobenzonitrile
- CAS Number:3939-09-1
- Molecular formula:C7H3F2N
- Molecular Weight:139.1
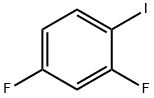
85118-02-1

3939-09-1
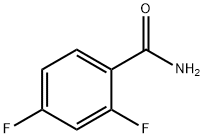
General procedure for the synthesis of 2,4-difluorobenzonitrile from 2,4-difluorobenzamide: In a 250 mL three-necked flask, 2,4-difluorobenzamide (23.0 g, 146.2 mmol) was dissolved in dry N,N-dimethylformamide (80 mL), and cooled to -15°C. The reaction was continued for 0.5 h at room temperature. Phosphorus oxychloride (112.1 g, 730.9 mmol) was slowly added dropwise and the reaction was kept for 0.5 h. The reaction was then continued for 7 h at room temperature. Upon completion of the reaction, the reaction solution was slowly poured into a vessel containing ice to induce precipitation of solids. Finally, 17.0 g of 2,4-difluorobenzonitrile was obtained in 83.4% yield.
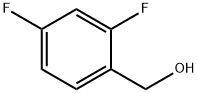
56456-47-4
199 suppliers
$5.00/1g

3939-09-1
304 suppliers
$10.00/5g
Yield:3939-09-1 72%
Reaction Conditions:
with 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical;ammonium acetate;oxygen;nitric acid;acetic acid at 50; under 760.051 Torr; for 12 h;Sealed tube;
Steps:
2.1 Experimental procedure for the aerobic conversion of benzyl alcohols to aromatic nitriles
General procedure: 0.5 mmol substrate, 1.5 mmol NH4OAc, 0.15 mmol TEMPO, 2 mL AcOH and 0.15 mmol HNO3 weresuccessively added to a dried 45 mL tube filled with 1atm oxygen. Then the reaction tube was sealed andplaced in a constant-temperature oil bath to perform the reaction for 12 h. Once the reaction time wasreached, the mixture was cooled to room temperature. Then the mixture was alkalized to pH 7-8 with sodiumhydroxide aqueous solution. GC analysis of organic phase provided the GC yields of the products.Subsequently, the crude product from another parallel experiment was purified by column chromatography,and identified by 1H-NMR, 1C-NMR or GC-MS
References:
Zhao, Bo;Ren, Yun-Lai;Ren, Fangping;Tian, Xinzhe;Zhao, Shuang [Letters in Organic Chemistry,2018,vol. 15,# 7,p. 627 - 632] Location in patent:supporting information

85118-02-1
134 suppliers
$10.00/1 g

3939-09-1
304 suppliers
$10.00/5g