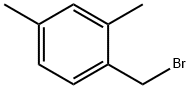
2,4-DIMETHYLBENZYL BROMIDE synthesis
- Product Name:2,4-DIMETHYLBENZYL BROMIDE
- CAS Number:78831-87-5
- Molecular formula:C9H11Br
- Molecular Weight:199.09
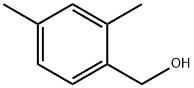
16308-92-2

78831-87-5
General procedure for the synthesis of 2,4-dimethylbenzyl bromide from (2,4-dimethylphenyl)methanol: 1. to a solution of diisopropyl ether (100 mL) of (2,4-dimethylphenyl)methanol (10.77 g) was slowly added phosphorus tribromide (4.90 mL) at 0 °C. 2. The reaction mixture was stirred at room temperature for 1 hour. 3. Upon completion of the reaction, water was added to the mixture and extracted with ethyl acetate. 4. The organic phases were combined and washed twice with water, saturated aqueous sodium bicarbonate and saturated brine in that order. 5. The organic layer was dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to give 2,4-dimethylbenzyl bromide as a colorless oil (15.5 g, quantitative yield). 6. The structure of the product was confirmed by 1H-NMR (CDCl3): δ 2.30 (3H, s), 2.37 (3H, s), 4.50 (2H, s), 6.96-6.99 (2H, m), 7.17-7.19 (1H, m).

16308-92-2
127 suppliers
$8.00/250mg

78831-87-5
66 suppliers
$75.00/1 g
Yield: 100%
Reaction Conditions:
with phosphorus tribromide in di-isopropyl ether at 0 - 35; for 1 h;
Steps:
60
Reference Example 60 1-(bromomethyl)-2,4-dimethylbenzene To a solution (100 mL) of (2,4-dimethylphenyl)methanol (10.77 g) obtained in Reference Example 58 in diisopropyl ether was added phosphorus tribromide (4.90 mL) at 0°C, and the reaction mixture was stirred at room temperature for 1 hr. Water was added to the reaction mixture and the mixture was extracted with ethyl acetate. The extract was washed twice with water, a saturated aqueous sodium hydrogen carbonate solution and saturated brine, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to give the title compound as a colorless oil (yield 15.5g, yield quantitative). 1H-NMR (CDCl3) δ: 2.30 (3H, s), 2.37 (3H, s), 4.50 (2H, s), 6.96-6.99 (2H, m), 7.17-7.19 (1H, m).
References:
Location in patent:Page/Page column 45

50-00-0
896 suppliers
$10.00/25g
108-38-3
383 suppliers
$10.60/50gm:

78831-87-5
66 suppliers
$75.00/1 g
824-55-5
93 suppliers
$44.00/1 g

78831-87-5
66 suppliers
$75.00/1 g