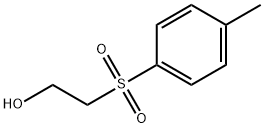
2-[(4-Methylphenyl)sulfonyl]ethanol synthesis
- Product Name:2-[(4-Methylphenyl)sulfonyl]ethanol
- CAS Number:22381-54-0
- Molecular formula:C9H12O3S
- Molecular Weight:200.25
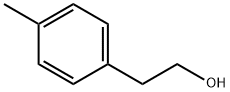
699-02-5
![2-[(4-Methylphenyl)sulfonyl]ethanol](/CAS/GIF/22381-54-0.gif)
22381-54-0
General procedure for the synthesis of 2-(p-toluenesulfonyl)ethanol from 4-methylphenylethanol: To a mixture of acetic acid (50 mL) and water (50 mL) of compound 2 (20 g, 0.119 mol) was added slowly and dropwise over a period of one-half hour 30% hydrogen peroxide (36.5 mL) under ice-bath conditions. Subsequently, the reaction mixture was refluxed for 20 minutes and then cooled to room temperature. Sodium bicarbonate was added to the reaction mixture to neutralize the solution, followed by ethyl acetate for extraction. The organic layer was separated, washed sequentially with saturated saline and deionized water, and finally concentrated under reduced pressure to give 22.9 g (96.3% yield) of target product 3 as a white solid. The structure of the product was confirmed by 1H NMR (270 MHz, CDCl3) and 13C NMR (67.9 MHz, CDCl3): 1H NMR (270 MHz, CDCl3) δ 2.46 (s, 3H), 3.33 (t, J = 5.4 Hz, 2H), 3.99 (t, J = 5.4 Hz, 2H), 7.39 (d, J = 8.0 Hz, 2H), 7.81 (d, J = 8.3 Hz, 2H); 13C NMR (67.9 MHz, CDCl3) δ 21.7, 56.4, 58.3, 128.0, 130.1, 136.0, 145.2.

699-02-5
178 suppliers
$24.00/1g
![2-[(4-Methylphenyl)sulfonyl]ethanol](/CAS/GIF/22381-54-0.gif)
22381-54-0
94 suppliers
$6.00/100mg
Yield: 96.3%
Reaction Conditions:
in water;acetic acid for 0.833333 h;Heating / reflux;
Steps:
3
2-(4-Tolysulfonyl)ethanol (3). To a solution of Compound 2 (20 g, 0.119 mol) in AcOH (50 ml) and H2O (50 ml) on ice bath was added hydrogen peroxide (30%, 36.5 ml) dropwise over half an hour. Then the mixture was refluxed for 20 min and cooled. NaHCO3 was added to the reaction mixture to neutralize this solution, followed by AcOEt. The organic layer was separated, washed with brine and water, concentrated on vacuum to give 22.9 g (96.3%) of 3 as a white solid. 1H NMR (270 MHz, CDCl3): ? 2.46 (s, 3H), 3.33 (t, J= 5.4 Hz, 2H), 3.99 (t, J= 5.4 Hz, 2H), 7.39 (d, J= 8.0 Hz, 2H ), 7.81 (d, J= 8.3 Hz, 2H). 13C NMR (67.9 MHz, CDCl3): ? 21.7, 56.4, 58.3, 128.0, 130.1, 136.0, 145.2.
References:
CHATTOPADHYAYA, Jyoti WO2007/64291, 2007, A1 Location in patent:Page/Page column 17
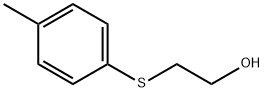
13290-16-9
10 suppliers
$32.29/250mg
![2-[(4-Methylphenyl)sulfonyl]ethanol](/CAS/GIF/22381-54-0.gif)
22381-54-0
94 suppliers
$6.00/100mg
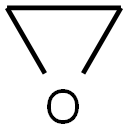
75-21-8
238 suppliers
$33.29/crm44609
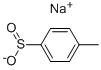
824-79-3
420 suppliers
$6.00/25g
![2-[(4-Methylphenyl)sulfonyl]ethanol](/CAS/GIF/22381-54-0.gif)
22381-54-0
94 suppliers
$6.00/100mg

824-79-3
420 suppliers
$6.00/25g

107-07-3
0 suppliers
$18.30/50ml
![2-[(4-Methylphenyl)sulfonyl]ethanol](/CAS/GIF/22381-54-0.gif)
22381-54-0
94 suppliers
$6.00/100mg

13290-16-9
10 suppliers
$32.29/250mg
![2-[(4-Methylphenyl)sulfonyl]ethanol](/CAS/GIF/22381-54-0.gif)
22381-54-0
94 suppliers
$6.00/100mg
![Ethanol, 2-[(4-methylphenyl)sulfinyl]-](/CAS/20210305/GIF/87943-26-8.gif)
87943-26-8
0 suppliers
inquiry