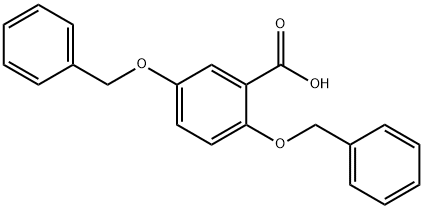
2,5-BIS-BENZYLOXY-BENZOIC ACID synthesis
- Product Name:2,5-BIS-BENZYLOXY-BENZOIC ACID
- CAS Number:67127-91-7
- Molecular formula:C21H18O4
- Molecular Weight:334.37
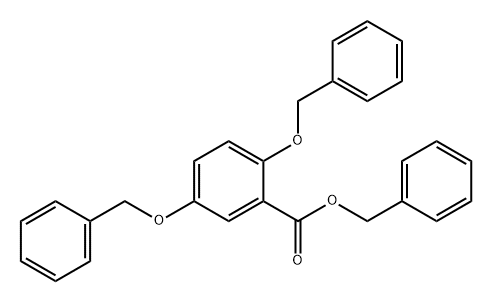
78283-37-1

67127-91-7
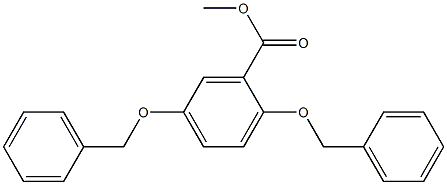
General procedure for the synthesis of 2,5-bis-benzyloxybenzoic acid from the compound (CAS: 78283-37-1): 1.0 g (2.4 mmol) of the ester 25 and 0.19 g (4.7 mmol) of sodium hydroxide were dissolved in 15 mL of a solvent mixture of methanol, dioxane, and water (1:1:1, v/v), and stirred at reflux for 14 hours. After completion of the reaction, it was cooled to 25 °C and most of the organic solvent was removed by rotary evaporator. The residue was diluted with water, acidified with 1N HCl to pH < 7 and subsequently extracted twice with ethyl acetate (50 mL). The organic phases were combined, washed sequentially with water and saturated saline, dried over anhydrous magnesium sulfate, and concentrated. The crude product was purified by column chromatography using hexane and ethyl acetate (1:1, v/v) as eluents to afford 0.72 g (91% yield) of the target compound 13.1H NMR (δ, ppm): 11.06 (s, 1H), 7.81 (d, J = 3.1 Hz, 1H), 7.43-7.32 (m, 10H), 7.17 (dd, J = 9.0, 3.1 Hz, 1H), 7.07 (d, J = 9.0 Hz, 1H), 5.26 (s, 2H), 5.08 (s, 2H); 13C NMR (δ, ppm): 165.3, 153.9, 151.9, 136.6, 134.6, 129.4, 129.4, 128.8, 128.4, 128.2, 127.8, 123.0, 119.0, 117.8, 115.1, 73.2, 70.9.

78283-37-1
1 suppliers
inquiry

67127-91-7
53 suppliers
$120.00/1g
Yield:67127-91-7 91%
Reaction Conditions:
with sodium hydroxide in 1,4-dioxane;methanol;water; for 14 h;Reflux;
Steps:
2,5-Dibenzyloxybenzoic acid (13)
A solution of 1.0 g (2.4 mmol) of ester 25 and 0.19 g (4.7 mmol) of sodium hydroxide in 15 mL of methanol, dioxane, and water (1:1:1) was stirred under reflux for 14 h, cooled to 25oC, and most of the organic solvents were removed from a rotary evaporator. The residue was diluted with water, acidified with 1 N HCl, extracted twice with ethyl acetate (50 mL), and the ethyl acetate layers were combined, washed with water and brine, dried (MgSO4), concentrated, and column chromatographed using a mixture of hexane and ethyl acetate (1:1) as eluent to give 0.72 g (91% yield) of compound 13.15 1H NMR δ 11.06 (s, 1 H), 7.81 (d, J = 3.1 Hz, 1 H), 7.43 - 7.32 (m, 10 H), 7.17 (dd, J = 9.0, 3.1 Hz, 1 H), 7.07 (d, J = 9.0 Hz, 1 H), 5.26 (s, 2 H), 5.08 (s, 2 H); 13C NMR δ 165.3, 153.9, 151.9, 136.6, 134.6, 129.4, 129.4, 128.8, 128.4, 128.2, 127.8, 123.0, 119.0, 117.8, 115.1, 73.2, 70.9.
References:
Thapa, Mahendra;Kim, Yunjeong;Desper, John;Chang, Kyeong-Ok;Hua, Duy H. [Bioorganic and Medicinal Chemistry Letters,2012,vol. 22,# 1,p. 353 - 356] Location in patent:supporting information; experimental part
106296-25-7
4 suppliers
inquiry

67127-91-7
53 suppliers
$120.00/1g
490-79-9
518 suppliers
$5.00/100mg

67127-91-7
53 suppliers
$120.00/1g
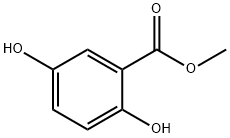
2150-46-1
243 suppliers
$6.00/1g

67127-91-7
53 suppliers
$120.00/1g

100-39-0
431 suppliers
$10.00/10g

67127-91-7
53 suppliers
$120.00/1g