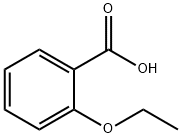
2-Ethoxybenzoic acid synthesis
- Product Name:2-Ethoxybenzoic acid
- CAS Number:134-11-2
- Molecular formula:C9H10O3
- Molecular Weight:166.17
6290-24-0

134-11-2
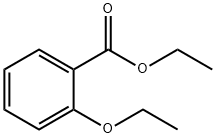
The general procedure for the synthesis of 2-ethoxybenzoic acid from ethyl o-ethoxybenzoate was as follows: potassium tert-butoxide (10 g, 890 mmol) was added batchwise to a stirred solution of ethyl 2-ethoxybenzoate (10 g, 240 mmol) in dimethyl sulfoxide (40 mL). The reaction mixture was heated to 70 °C in a water bath and maintained at this temperature for 2 h. The progress of the reaction was monitored by thin layer chromatography (TLC) using hexane-ethyl acetate (8:2) as a spreading agent. Upon completion of the reaction, the reaction mixture was cooled to 10 °C and poured into ice water. Subsequently, the reaction mixture was acidified with 5% dilute hydrochloric acid. The precipitated solid was filtered and washed thoroughly with distilled water. The crude product was recrystallized in hexane (50 mL) to give a final off-white solid 2-ethoxybenzoic acid (7.6 g, 80% yield).
Yield:134-11-2 98.31%
Reaction Conditions:
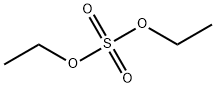
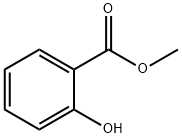
Stage #1:diethyl sulfate;methyl salicylate with potassium hydroxide in ethanol at 15; for 6 h;Large scale;
Stage #2: with water;sodium hydroxide at 65; for 6 h;Large scale;Temperature;
Steps:
1-3
Put 5% of 95% edible ethanol into a 1000L reaction kettle.Under stirring and cooling conditions,60Kg solid KOH (90% purity) was added in batches, and methyl salicylate (99.5% purity, medical grade) 152Kg was added dropwise at 15 °C.And control the drop acceleration and temperature drop conditions, so that the temperature is always around 15 °C.After the addition, at 15 ° C, 162 Kg of diethyl sulfate was added dropwise to control the dropping rate and temperature drop conditions, so that the temperature in the kettle was 15 ° C, and after adding diethyl sulfate, the reaction was about 6 h.The pH was measured, and when pH was <11, KOH 6.1 Kg was added, and the reaction was continued at 15 ° C until pH = 6.Filtration, removal of solids (solids are white flaky crystals), the filtrate is distilled to recover ethanol, and the recovered ethanol is 353Kg.Obtaining a colorless oil, washing with water to remove inorganic salts (mainly potassium sulfate),The oil is in a 1000L reactor,Add 665 Kg of water and 53.2 Kg of NaOH, and stir to raise the temperature at 65 ° C for 6 h.A colorless, clear, transparent liquid of pH = 14 was obtained.After the reaction solution was cooled, hydrochloric acid was added to adjust pH=4.5 to obtain a colorless transparent oil. The organic layer was separated and washed twice with water.Distillation under reduced pressure gave 163.2 Kg of o-ethoxybenzoic acid.The yield was 98.31%, and the purity was 99.73%.
References:
Zhang Yuanhao CN109503370, 2019, A Location in patent:Paragraph 0029; 0036-0040; 0041-0045; 0046-0050

6290-24-0
132 suppliers
$45.00/100mg

134-11-2
396 suppliers
$6.00/5g
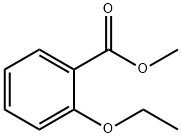
3686-55-3
117 suppliers
$8.00/1g

134-11-2
396 suppliers
$6.00/5g
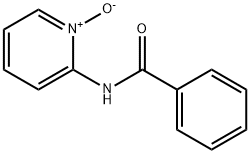
14178-42-8
5 suppliers
inquiry

134-11-2
396 suppliers
$6.00/5g
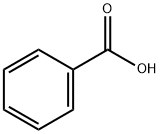
65-85-0
1436 suppliers
$10.00/25 g

134-11-2
396 suppliers
$6.00/5g