
2,5-Di(4-formylphenyl)thiophene synthesis
- Product Name:2,5-Di(4-formylphenyl)thiophene
- CAS Number:193903-62-7
- Molecular formula:C18H12O2S
- Molecular Weight:292.35
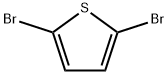
3141-27-3
309 suppliers
$5.00/10g
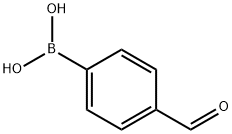
87199-17-5
433 suppliers
$8.00/5g

193903-62-7
22 suppliers
inquiry
Yield:193903-62-7 86%
Reaction Conditions:
Stage #1: 2,5-dibromothiophen;4-formylphenylboronic acid,with potassium carbonate in N,N-dimethyl-formamide; for 0.5 h;Inert atmosphere;Suzuki Coupling;
Stage #2: with tetrakis(triphenylphosphine) palladium(0) in d7-N,N-dimethylformamide at 100; for 6 h;Inert atmosphere;Suzuki Coupling;
Steps:
1
(1)2,5-Bis(4-formylphenyl)thiophene BFT: First, 2,5-dibromothiophene (0.484 g, 2 mmol),4-formylphenylboronic acid (0.72 g, 4.8 mmol),K2CO3 (0.696g, 1N) and N,N-dimethylformamide (5mL) were mixed for 30 minutes under N2 atmosphere.Then put tetrakis(triphenylphosphine)palladium (0.115 g, 0.1 mmol),The reaction was stirred at 100 ° C for 6 hours.The reaction is completed,Natural cooling,The resulting orange suspension was filtered and extracted with chloroform.At last,Product column chromatography,The target product was a brown solid, 0.51 g.The yield was 86%.
References:
CN108484588,2018,A Location in patent:Paragraph 0043; 0044

55368-38-2
19 suppliers
inquiry

193903-62-7
22 suppliers
inquiry
26076-46-0
90 suppliers
$59.00/5g
1122-91-4
702 suppliers
$9.00/10g

193903-62-7
22 suppliers
inquiry
126747-14-6
408 suppliers
$6.00/1g

193903-62-7
22 suppliers
inquiry