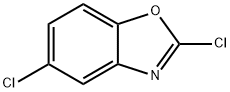
2,5-Dichlorobenzooxazole synthesis
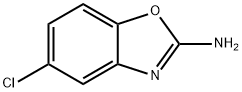
- Product Name:2,5-Dichlorobenzooxazole
- CAS Number:3621-81-6
- Molecular formula:C7H3Cl2NO
- Molecular Weight:188.01
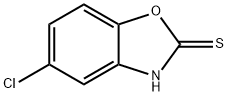
22876-19-3

3621-81-6
General procedure for the synthesis of 2,5-dichlorobenzoxazole from 5-chloro-2-mercaptobenzoxazole: Thionyl chloride (885 g, 7.5 mol) and N,N-dimethylformamide (270 ml) were added drop-wise to a solution of 5-chloro-2-mercaptobenzoxazole (540 g, 2.903 mol) in dichloromethane (5 L) at 5-10°C. The reaction mixture was stirred until a clarified solution was formed, followed by continued stirring for 4 hours at room temperature. Upon completion of the reaction, the reaction solution was slowly poured into 4 L of ice water and neutralized with sodium bicarbonate to pH neutral within 1 hour. The aqueous phase was extracted with dichloromethane (2.5 L x 2) and the combined organic layers were washed with saturated brine and dried over anhydrous sodium sulfate. The organic phase was concentrated under reduced pressure to give the crude product. The crude product was washed with n-hexane (2L×2) at -20°C, filtered and dried to give a yellow liquid 2,5-dichlorobenzoxazole (496.8 g, 91.0% yield).

22876-19-3
161 suppliers
$13.00/5g

3621-81-6
174 suppliers
$8.00/1g
Yield:3621-81-6 91%
Reaction Conditions:
with thionyl chloride;N,N-dimethyl-formamide in dichloromethane at 5 - 20; for 4 h;
Steps:
1.3 Step 3: Synthesis of 2,5-dichlorobenzoxazole.
Thionyl chloride (885 g, 7.5 mol)And N, N-dimethylformamide (270 ml) were added to a solution of 5-chloro-2-mercaptobenzoxazole (540 g, 2.903 mol) in dichloromethane (5 L) at 5-10 ° C.Stirred until a clear solution was formed and the reaction solution was stirred at room temperature for 4 hours.After completion of the reaction, the reaction solution was poured into 4 L of ice water, neutralized to neutral with sodium bicarbonate in 1 hour, and extracted with dichloromethane (2.5 L * 2).The organic layer was washed with brine, dried over sodium sulfate and dried to give the crude product. The crude product was washed with n-hexane (2 L * 2) at -20 ° C, filtered and dried to give yellow liquid 2,5-dichlorobenzo (496.8 g, 91.0%).
References:
Shanghai ZaiQi Bio-Tech Co., Ltd.;Wang, Zhiguo;Song, Yanhong;Ma, Xiujuan;Tian, Beibei;Li, Shijiang;Li, Chao;Li, Qiang;Li, Tao CN106478537, 2017, A Location in patent:Paragraph 0027; 0028; 0039; 0040; 0050; 0051; 0065; 0066
95-85-2
400 suppliers
$13.00/25g

3621-81-6
174 suppliers
$8.00/1g

22876-19-3
161 suppliers
$13.00/5g

3621-81-6
174 suppliers
$8.00/1g
89-64-5
13 suppliers
$27.60/25g

3621-81-6
174 suppliers
$8.00/1g
61-80-3
163 suppliers
$5.00/10mg

3621-81-6
174 suppliers
$8.00/1g