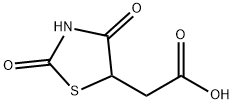
2-[(5S)-2,4-dioxothiazolidin-5-yl]acetate synthesis
- Product Name:2-[(5S)-2,4-dioxothiazolidin-5-yl]acetate
- CAS Number:875-97-8
- Molecular formula:C5H5NO4S
- Molecular Weight:175.16
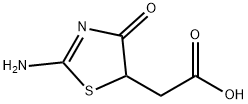
33176-41-9
25 suppliers
$63.61/10mgs:
![2-[(5S)-2,4-dioxothiazolidin-5-yl]acetate](/CAS/GIF/875-97-8.gif)
875-97-8
43 suppliers
$109.56/1gm:
Yield:875-97-8 80%
Reaction Conditions:
with sulfuric acid;water for 3 h;Reflux;
Steps:
Synthesis of 2,4-dioxo-5-thiazolidineacetic acid (4)
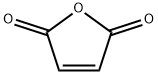
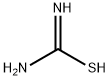
A mixture of thiourea (76.12 g, 1 mol) and maleic anhydride (98.00 g, 1 mol) was refluxed in water (400 mL). After 3 h of heating the reaction mass was allowed to cool at rt and the generated solid mass was filtered and washed with water. The afforded intermediate 2-imino-4-oxo-5-thiazolidineacetic acid 3 (30 g) was then hydrolyzed using 20% aqueous H2SO4 (250 mL) under reflux for 3 h. The solid obtained after cooling the hot reaction mass to rt was filtered and dried.2-imino-4-oxo-5-thiazolidineacetic acid (3) Yield 92%, Lit.19 mp 250-251 °C, Obsd mp 251-252 °C, 1H NMR (300 MHz, DMSO-d6, δ ppm):12.60 (br s, 1H, -COOH, D2O exchangeable), 8.18-8.87 (br s, 2H, -NH, D2O exchangeable), 4.30-4.33 (t,J = 7.60 Hz, 1H, methine proton, TZD-5-H), 3.05 (dd, J = 8.00 Hz, J = 3.60 Hz, 2H, CH2). EI MS (m/z): 174. 2,4-dioxo-5-thiazolidineacetic acid (4) Yield 80%, Lit.19 mp 166-167 °C, Obsd mp 167-168 °C, 1H NMR (300 MHz, DMSO-d6, δ ppm): 11.99 (br s, 1H, -COOH, D2O exchangeable), 7.10 (br s, 1H, NH, D2O exchangeable), 4.16 (t, J = 7.10 Hz, 1H methine proton, TZD-5-H), 3.07 (dd, J = 8.10 Hz, J = 5.10 Hz, 2H, CH2). DART MS (ESI+, m/z): 176 (M+).
References:
Jawale, Dhanaji V.;Pratap, Umesh R.;Rahuja, Neha;Srivastava, Arvind K.;Mane, Ramrao A. [Bioorganic and Medicinal Chemistry Letters,2012,vol. 22,# 1,p. 436 - 439] Location in patent:experimental part
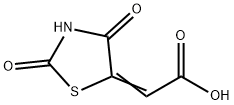
5374-29-8
17 suppliers
$60.00/100mg
![2-[(5S)-2,4-dioxothiazolidin-5-yl]acetate](/CAS/GIF/875-97-8.gif)
875-97-8
43 suppliers
$109.56/1gm: