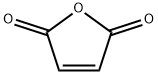
Maleic anhydride synthesis
- Product Name:Maleic anhydride
- CAS Number:108-31-6
- Molecular formula:C4H2O3
- Molecular Weight:98.06
Fumaric and maleic acids both give maleic anhydride on heating. Fumaric acid must first be heated to a higher temperature to effect its conversion to maleic acid prior to its dehydration.
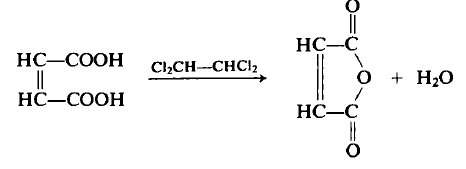
![2-Furancarboxaldehyde, 5-[(formyloxy)methyl]-](/CAS/20210305/GIF/102390-86-3.gif)
102390-86-3
1 suppliers
inquiry

108-31-6
884 suppliers
$13.47/50g
Yield:108-31-6 96.35%
Reaction Conditions:
with manganese(IV) oxide;dipotassium peroxodisulfate in water at 90; for 5 h;Catalytic behavior;Reagent/catalyst;Temperature;Solvent;
Steps:
1-22 Example 19:
0.50g 5-formyloxymethylfurfural (FMF), 0.88g of potassium persulfate, Manganese dioxide 0.03g, Copper nitrate 0.02g, 25mL of dichlorotoluene, 5mL of deionized water is added to the 50mL reactor, Heat to 90°C with magnetic stirring and react for 5h. The mixture in the reaction system was cooled to room temperature, and the yield of maleic anhydride (MA) was 96.35%.
References:
CN111187237,2020,A Location in patent:Paragraph 0017-0061
67-47-0
612 suppliers
$5.00/100mg

108-31-6
884 suppliers
$13.47/50g
![5-HYDROXY-2[5H]-FURANONE](/CAS/GIF/14032-66-7.gif)
14032-66-7
50 suppliers
$315.00/100mg

108-31-6
884 suppliers
$13.47/50g
98-01-1
508 suppliers
$22.00/25g

108-31-6
884 suppliers
$13.47/50g
106-97-8
114 suppliers
$40.00/1mL

108-31-6
884 suppliers
$13.47/50g