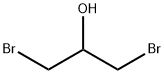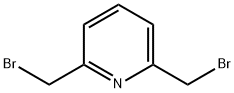
2,6-BIS(BROMOMETHYL)PYRIDINE synthesis
- Product Name:2,6-BIS(BROMOMETHYL)PYRIDINE
- CAS Number:7703-74-4
- Molecular formula:C7H7Br2N
- Molecular Weight:264.95
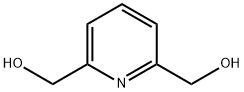
1195-59-1

7703-74-4
Example 15 Synthesis of 2,6-bis(bromomethyl)pyridine. To pyridine-2,6-dimethanol (2 g, 14 mmol) was slowly added 60% hydrobromic acid (15 mL). The reaction mixture was heated to reflux at 125 °C for 6 h and subsequently cooled to room temperature. The reaction residue was dissolved in water (50 mL) to form a yellow solution. To this solution saturated sodium bicarbonate solution was added dropwise until the pH reached 8. The resulting aqueous solution was extracted with dichloromethane (4 x 50 mL) and the organic layers were combined and dried over anhydrous sodium sulfate. The solvent was removed by rotary evaporation and the resulting crude product was purified by fast column chromatography (ethyl acetate/hexane, 1:9 to 1:4 gradient elution) to afford 2,6-bis(bromomethyl)pyridine (3.5 g, 96% yield) as a white solid. The structure of the product was analyzed by 1H NMR (500 MHz, CDCl3) δ 4.53 (s, 4H), 7.36-7.38 (d, J = 8.0 Hz, 2H), 7.68-7.71 (dd, J = 7.5,8.0 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 33.8,123.1,138.4, 157.0; LC-TOF (M + H+) calculated value C7H8Br2N 263.90235, measured value 263.90193 confirmed.

1195-59-1
381 suppliers
$6.00/1g

7703-74-4
149 suppliers
$52.00/1g
Yield:7703-74-4 96%
Reaction Conditions:
Stage #1: 2.6-bis(hydroxymethyl)pyridinewith hydrogen bromide in water at 125;
Stage #2: with sodium hydrogencarbonate in water; pH=8;
Steps:
15
Example 15 2,6-Bis(bromomethyl)pyridine. To pyridine-2,6-diyldimethanol (2 g, 14 mmol) was added 60% HBr (15 mL) slowly. The reaction was heated at 125° C. for 6 h then cooled to room temperature. The resulting residue was dissolved in H2O (50 mL) to give a yellow solution. To this solution was added saturated NaHCO3 to pH 8. The resulting aqueous solution was extracted with CH2Cl2 (4×50 mL), and the combined organic layers were dried over Na2SO4. The solvent was removed by rotary evaporation, and the resulting material was purified by flash column chromatography (EtOAc/hexanes, 1:9-1:4) to yield 2,6-bis(bromomethyl)pyridine (3.5 g, 96%) as a white solid: 1H NMR (500 MHz, CDCl3) δ 4.53 (s, 4H), 7.36-7.38 (d, J=8.0 Hz, 2H), 7.68-7.71 (dd, J=7.5, 8.0 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 33.8, 123.1, 138.4, 157.0; LC-TOF (M+H+) calcd for C7H8Br2N 263.90235, found 263.90193.
References:
US2010/203613,2010,A1 Location in patent:Page/Page column 10
108-48-5
489 suppliers
$9.90/190μL

7703-74-4
149 suppliers
$52.00/1g
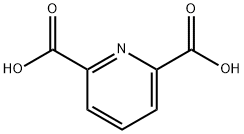
499-83-2
636 suppliers
$6.00/10g

7703-74-4
149 suppliers
$52.00/1g
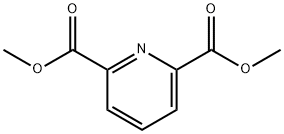
5453-67-8
298 suppliers
$6.00/5g

7703-74-4
149 suppliers
$52.00/1g