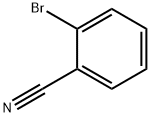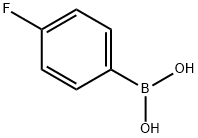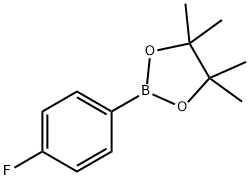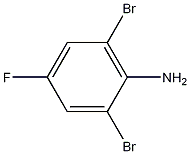
2,6-dibromo-4-fluoroaniline synthesis
- Product Name:2,6-dibromo-4-fluoroaniline
- CAS Number:89346-55-4
- Molecular formula:C6H4Br2FN
- Molecular Weight:268.9091
Yield:89346-55-4 93%
Reaction Conditions:
with Ph2P(CH2CH2O)22CH3;triethylamine;palladium dichloride in water at 80; for 2 h;Inert atmosphere;Suzuki coupling;
Steps:
General Procedure for the Suzuki Reaction of Aryl Bromides with Arylboronic Acids
General procedure: A solution of PdCl2 (0.09 mg, 0.0005 mmol) and ligand L (1.2 mg, 0.001 mmol) in deoxygenated H2O (1 mL) was stirred at room temperature for 30 min under nitrogen. Et3N (1 mmol, 101 mg), aryl bromide (0.5 mmol), arylboronic acid (0.75 mmol) were then successively added. The reaction mixture was heated in oil bath under nitrogen with magnetic stirring. After cooling to room temperature, the reaction mixture was added to brine (15 mL) and extracted three times with diethyl ether (3×15 mL). The solvent was concentrated under vacuum and the product was isolated by short chromatography on a silica gel (200-300 mesh) column.
References:
Liu, Ning;Liu, Chun;Jin, Zilin [Journal of Organometallic Chemistry,2011,vol. 696,# 13,p. 2641 - 2647] Location in patent:supporting information; experimental part