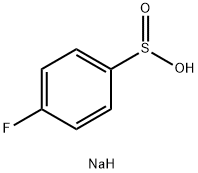
4-FLUOROBENZENESULFINIC ACID SODIUM SALT synthesis
- Product Name:4-FLUOROBENZENESULFINIC ACID SODIUM SALT
- CAS Number:824-80-6
- Molecular formula:C6H6FNaO2S
- Molecular Weight:184.16
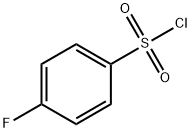
349-88-2

824-80-6
GENERAL STEPS: In a 250 mL single-necked round-bottomed flask, 4-fluorobenzenesulfonyl chloride (48.5 mmol, 9.4 g, 1.0 eq.), sodium sulfite (97 mmol, 12.2 g, 2.0 eq.), and sodium bicarbonate (97 mmol, 8.1 g, 2.0 eq.) were added in sequence, followed by 60 mL of water. A reflux condenser was installed and the reaction was stirred in an oil bath at 80 °C for 4 hours. Upon completion of the reaction, the water was removed by distillation under reduced pressure and the residue was dissolved in methanol and filtered. The filtrate was concentrated by rotary evaporator to remove methanol, and 10.2 g of sodium p-fluorobenzenesulfinate was obtained as a white solid product with 99% yield.

349-88-2
313 suppliers
$6.00/5g

824-80-6
120 suppliers
$5.00/1g
Yield: 99%
Reaction Conditions:
with sodium hydrogencarbonate;sodium sulfite in water at 80; for 4 h;
Steps:
3
Take 250mL of mono vial,P-fluorobenzenesulfonyl chloride (48.5 mmol, 9.4 g, 1.0 equiv) was added,Sodium sulfite (97 mmol, 12.2 g, 2.0 equiv),Sodium bicarbonate (97 mmol, 8.1 g, 2.0 equiv)Add 60 mL of water,Equipped with reflux condenser,80 ° C for 4 h.The reaction ends,Dry water,Add methanol dissolved to filter,And then spin dry methanol to white solid 10.2g,Yield 99%.
References:
Chinese Academy Of Sciences Shanghai Organic Chemistry Institute;Shen Qilong;Wu Jiang;Zhao Qunchao CN107033046, 2017, A Location in patent:Paragraph 0192; 0193; 0194
460-00-4
635 suppliers
$6.00/10g

824-80-6
120 suppliers
$5.00/1g
462-06-6
432 suppliers
$10.00/5g

824-80-6
120 suppliers
$5.00/1g
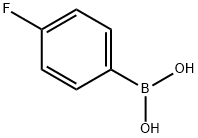
1765-93-1
552 suppliers
$9.00/1g

824-80-6
120 suppliers
$5.00/1g
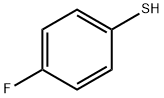
371-42-6
319 suppliers
$6.00/5g

824-80-6
120 suppliers
$5.00/1g