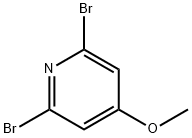
2,6-Dibromo-4-methoxypyridine synthesis
- Product Name:2,6-Dibromo-4-methoxypyridine
- CAS Number:117873-72-0
- Molecular formula:C6H5Br2NO
- Molecular Weight:266.92
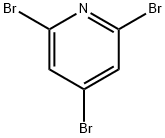
2408-70-0

124-41-4

117873-72-0
Reaction of 2,4,6-tribromopyridine (5) and sodium methanol (1.2 eq.) under methanol reflux conditions afforded 2,6-dibromo-4-methoxypyridine (6) in 80% yield. Subsequently, the compound (6) was treated with n-butyllithium (1.2 eq.) at -78 °C and reacted with pivaleronitrile (1.2 eq.) for 150 min, followed by refluxing in two conventional sulfuric acids for 2 h to afford the keto isomer (7) in 86% yield. Compound (7) was converted to optically active alcohol (8) in 93% yield and 90% optical purity by hydrogen transfer type asymmetric reduction catalyzed by asymmetric ruthenium catalyst RuCl[(S,S)-Tddpen] (p-isopropylbenzene, 0.01 equiv.) using formic acid (4.3 equiv.) and triethylamine (2.5 equiv.). Next, compound (8) was converted to a camphor ester using a chloride, processed for optical splitting by recrystallization (75% yield, diastereoisomer ratio = 99/<1), and saponified again to give an almost optically pure alcohol (7, quantitatively). Finally, homogeneous coupling of compound (7) using palladium catalyst [PdCl2(PhCN)2-TDAE] afforded the pyridine isomer (9) (chemical formula 5) in 36% yield (diastereoisomer ratio = >99.5/<0.5).

2408-70-0
108 suppliers
$17.00/250mg

124-41-4
726 suppliers
$12.00/25g

117873-72-0
85 suppliers
$15.00/250mg
Yield: 80%
Reaction Conditions:
in methanol for 2.5 h;Heating / reflux;
Steps:
2
2,6-Dibromo-4-methoxy pyridine (6) was obtained in 80% yield when 2,4,6-tribromopyridine (5) was allowed to react with sodium methoxide (1.2 eq) in refluxing methanol. The compound (6) was treated using n-butyl lithium (1.2 eq.), was allowed to react with pivalonitrile (1.2 eq.) for 150 minutes at -78°C and was refluxed for two hours in two normal sulfuric acid to yield ketone isomer (7) in 86% yield. An optically active alcohol (8) was obtained in 93% yield and in 90% ee optical purity from compound (7) through hydrogen transfer type asymmetric reduction of formic acid (4.3 eq.) and triethylamine (2.5 eq.) using the asymmetric ruthenium catalyst (RuCl[(S,S]-Tsdpen)(p-cumene), 0.01 eq.) as the catalyst. The compound (8) was converted to a camphor ester using the acid chloride, an optical resolution process was conducted using re-crystallization (75% yield, diastereomer ratio = >99/<1) and saponified again to obtain an almost optically pure alcohol (7, quant.). The compound (7) was subjected to homo coupling using a palladium catalyst [PdCl2(PhCN)2-TDAE] to yield a pyridine isomer (9) (Chemical formula 5) in 36% yield (diastereomer ratio =>99.5/<0.5).
References:
Japan Science and Technology Agency EP1724251, 2006, A1 Location in patent:Page/Page column 5; 9

124-41-4
726 suppliers
$12.00/25g
175422-04-5
128 suppliers
$51.00/1 g

117873-72-0
85 suppliers
$15.00/250mg

67-56-1
789 suppliers
$7.29/5ml-f

175422-04-5
128 suppliers
$51.00/1 g

117873-72-0
85 suppliers
$15.00/250mg
437710-07-1
1 suppliers
inquiry

117873-72-0
85 suppliers
$15.00/250mg

175422-04-5
128 suppliers
$51.00/1 g

117873-72-0
85 suppliers
$15.00/250mg