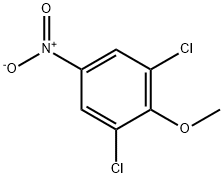
2,6-DICHLORO-4-NITROANISOLE synthesis
- Product Name:2,6-DICHLORO-4-NITROANISOLE
- CAS Number:17742-69-7
- Molecular formula:C7H5Cl2NO3
- Molecular Weight:222.03
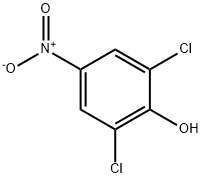
618-80-4

74-88-4

17742-69-7
GENERAL STEPS: To a stirred solution of 2,6-dichloro-4-nitrophenol (6.0 g, 28.84 mmol) in anhydrous DMF (60 mL) was added anhydrous K2CO3 (15.9 g, 115.36 mmol) and iodomethane (8.18 g, 57.69 mmol). The reaction mixture was stirred at 0°C and then gradually warmed up to 60°C and kept for 6 hours. After completion of the reaction, the mixture was cooled to room temperature and slowly poured into ice water to quench the reaction. The precipitated solid was collected by filtration and dried under vacuum to obtain the crude product. The crude product was purified by column chromatography using petroleum ether: ethyl acetate (85:15) as eluent to give 2.5 g (39.0% yield) of 1,3-dichloro-2-methoxy-5-nitrobenzene as an off-white solid. The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ 8.22 (s, 2H), 4.01 (s, 3H).
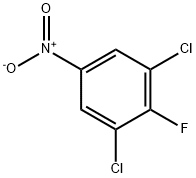
3107-19-5
185 suppliers
$5.00/1g

124-41-4
731 suppliers
$12.00/25g

17742-69-7
138 suppliers
$28.00/1 g
Yield: 98%
Reaction Conditions:
in methanol at 0 - 20; for 1.5 h;
Steps:
30.1; 31.1 Step 1: Example 30b
A mixture of Example 30a (2.1 g, 10 mmol) in MeOH (25 mL) was slowly added NaOMe (810 mg, 15 mmol) at 0°C. The reaction mixture was stirred at r.t. for 1.5 h. Water (50 mL) was added, and the mixture was extracted with EtOAc (100 mL*2). The combined organic layers were washed by brine (50 mL), separated, dried over Na2S04 and filtered. The solvent was removed in reduced pressure to give the desired product Example 30b (2.18 g, yield 98%) as a white solid. *HNMR (400 MHz, CDCl3) d 8.21 (s, 2H), 4.01 (s, 3H).
References:
FRONTHERA U.S. PHARMACEUTICALS LLC;JIN, Bohan;DONG, Qing;HUNG, Gene WO2019/240938, 2019, A1 Location in patent:Paragraph 00392; 00400

618-80-4
202 suppliers
$17.30/5g

74-88-4
357 suppliers
$15.00/10g

17742-69-7
138 suppliers
$28.00/1 g
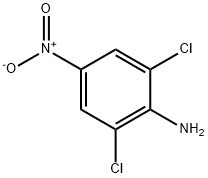
99-30-9
388 suppliers
$9.00/25g

17742-69-7
138 suppliers
$28.00/1 g
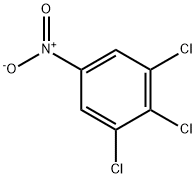
20098-48-0
211 suppliers
$6.00/1g

124-41-4
731 suppliers
$12.00/25g

17742-69-7
138 suppliers
$28.00/1 g
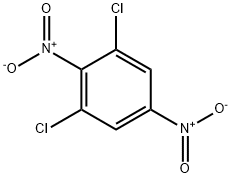
13633-34-6
0 suppliers
inquiry

124-41-4
731 suppliers
$12.00/25g

17742-69-7
138 suppliers
$28.00/1 g