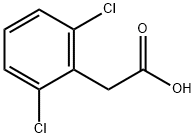
2,6-Dichlorophenylacetic acid synthesis
- Product Name:2,6-Dichlorophenylacetic acid
- CAS Number:6575-24-2
- Molecular formula:C8H6Cl2O2
- Molecular Weight:205.04
the method comprises the following steps: 2, 6-dichlorotoluene is used as a raw material, and is catalyzed by a complex catalyst formed by transition metal and a ligand (wherein, a transition metal catalyst precursor is preferably palladium chloride, an oxidant is preferably TBP (tert-butyl peroxy ether), and a ligand is preferably Xantphos (4, 5-bis (diphenylphosphino) -9, 9-dimethyl xanthene)) in the presence of an alcohol and a catalyst and an oxidant to obtain 2, 6-dichlorophenylacetic acid, and the ethyl 2, 6-dichlorophenylacetate is prepared by hydrolysis and acidification, wherein the total yield is 68.4%. See patent document US2013303798, the reaction procedure is described as synthetic route 1.
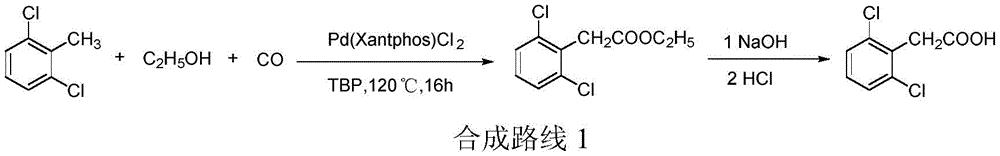
In the above synthetic route 1, the preparation process of the intermediate ethyl 2, 6-dichlorophenylacetate requires the use of carbon monoxide for high-temperature and high-pressure reaction, and has poor operation safety, high equipment requirement, high cost, and is not favorable for cost reduction and green production of 2, 6-dichlorophenylacetic acid.
3215-64-3
228 suppliers
$10.00/5g

6575-24-2
298 suppliers
$8.00/5g
Yield:6575-24-2 83%
Reaction Conditions:
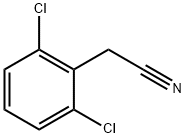
Stage #1:2,6-dichlorophenylacetonitrile with potassium hydroxide;water in ethanol at 80; for 20 h;
Stage #2: with hydrogenchloride in ethanol; pH=3
Steps:
A
A solution of (2,6-dichloro-phenyl)-acetonitrile (Intermediate A1) (commercially available at Aldrich) (18.6 g, 100 mmol) in ethanol (40 mL) and water (50 mL) was treated with KOH (30 g) and the mixture was heated to 80° C. for 20 h. The mixture was quenched with HCl until pH 3. The product was extracted with chloroform (5×50 mL). The extracts were combined, dried over MgSO4, filtered and evaporated to dryness. The product was (2,6-dichloro-phenyl)-acetic acid, 17 g (83%). A solution of (2,6-dichloro-phenyl)-acetic acid (10 g, 49 mmol) in benzene (200 mL) was treated with oxalyl chloride (32 mL, 2M in dichloromethane) followed by a few drops of dimethyl formamide. The mixture was allowed to stir for 3.5 h at rt. The solvent was removed under vacuum to give 11.5 g of (2,6-dichloro-phenyl)-acetyl chloride (Intermediate A2). The acid chloride, Intermediate A2 (6 g, 26.8 mmol) was added via pipette to a solution of diazomethane in ether (30 mmol) (generated from Diazald by standard Aldrich diazomethane kit) at 0° C. After 35 m, HBr (conc.) (10 mL) was added at 0° C. This was allowed to react for 35 m. The ether was removed and the mixture was neutralized with sodium bicarbonate solution. The organic layer was removed and dried over MgSO4. The mixture was filtered and concentrated under reduced pressure to give 1-bromo-3-(2,6-dichloro-phenyl)-propan-2-one (Intermediate A3) (5 g, 66%). 1-Bromo-3-(2,6-dichloro-phenyl)-propan-2-one (Intermediate A3) (2.5 g) was heated in formamide for 2 h at 180° C. and 150° C. for 1 additional h. Water (50 mL) was added and the mixture was extracted with chloroform (4×50 mL). The solution was washed with brine (1×30 mL), dried over MgSO4, filtered and evaporated to dryness. The residue was purified by chromatography on silica gel with 5% NH3-MeOH: CH2Cl2 to give the product 5-(2,6-dichloro-benzyl)-1H-imidazole (Intermediate A4), 400 mg. A solution of 5-(2,6-dichloro-benzyl)-1H-imidazole (Intermediate A4) (0.34 g, 1.5 mmol) in THF (6 mL) and water (6 mL) was treated with NaHCO3 (1.2 g) at rt for 10 m. Phenyl chlorothionoformate (0.60 mL, 4.3 mmol) was added and stirring was continued for 3 h. The mixture was diluted with water (10 mL) and extracted with ether (4×15 mL). The organic portions were combined, dried over MgSO4, filtered and freed of solvent. The residue was dissolved in MeOH (6 mL) and treated with NEt3 (0.6 mL) for 18 h. The solvent was removed under vacuum and the product was washed on a glass frit with CH2Cl2 to give a white solid 4-(2,6-dichloro-benzyl)-1,3-dihydro-imidazole-2-thione (Compound 1) in 50% yield. 1H NMR (300 MHz, DMSO-d6): δ 12.0 (s, 1H), 11.7 (s, 1H), 7.50 (d, J=5.1 Hz, 2H), 7.35 (t, J=6.0 Hz, 1H), 6.04 (s, 1H), 3.98 (s, 2H).
References:
Allergan, Inc. US2006/69142, 2006, A1 Location in patent:Page/Page column 9-10
90793-64-9
27 suppliers
$339.00/5g

6575-24-2
298 suppliers
$8.00/5g
2014-83-7
306 suppliers
$9.00/5g

6575-24-2
298 suppliers
$8.00/5g