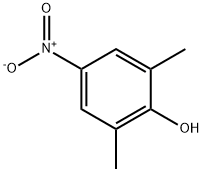
2,6-DIMETHYL-4-NITROPHENOL synthesis
- Product Name:2,6-DIMETHYL-4-NITROPHENOL
- CAS Number:2423-71-4
- Molecular formula:C8H9NO3
- Molecular Weight:167.16
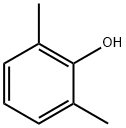
576-26-1

2423-71-4
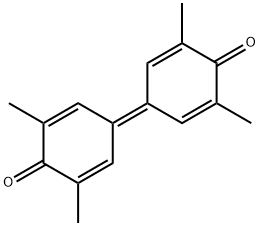
4906-22-3
General method: A solid mixture of 2,6-dimethylphenol (1-3 eq.) and Bi(NO3)3-3H2O (1 eq.) or Fe(NO3)3-9H2O (1 eq.) was mixed with acetone (10 mL/mmol). The reaction mixture was stirred in air at room temperature or under reflux conditions for 2-24 hours (see Tables 1 and 2). Upon completion of the reaction, the insoluble material was removed by filtration through a diatomaceous earth pad and the residue was washed with acetone (~5 mL/mmol). The filtrate was treated with NaHCO3 (0.1 g/mmol) until CO2 release ceased. The insoluble material was removed by filtration again, followed by concentration under reduced pressure in a water bath at 25-35°C to remove the solvent. The nitration products were separated or purified by silica gel column chromatography to give pure phenolic compounds. All products were characterized by 1H NMR, 13C NMR and IR and identified by comparison with spectral data and melting points reported in the literature.

576-26-1
344 suppliers
$10.00/25g

2423-71-4
114 suppliers
$7.00/250mg

4906-22-3
38 suppliers
$144.00/1g
Yield:2423-71-4 65% ,4906-22-3 17%
Reaction Conditions:
with bismuth (III) nitrate pentahydrate in acetone at 20; for 20 h;Reagent/catalyst;
Steps:
1.2. General procedure for the nitration of phenolic compounds
General procedure: To a solid mixture of phenol (1-3 equiv) and Bi(NO3)35H2O (1 equiv) or Fe(NO3)39H2O (1 equiv) was added acetone (10 ml/mmol). The resulting mixture was stirred at room temperature under air or at reflux for 2-24 hours, Tables 1 and 2. When the reaction was completed the insoluble materials were filtered off using a pad of Celite and the residue was washed by acetone (ca. 5 ml/mmol). The filtrate was treated by NaHCO3 (0.1 g/mmol) until evolution of CO2 stopped. Insoluble material was filtered off again, and the solvent was removed under vacuum in a water bath 25-35°C. The nitrated products were separated or purified using silica gel chromatography, to give pure phenolic compounds. All products were characterized by 1H NMR,13C NMR and IR and were identified by comparison of the spectral data and melting points with those reported in literature and characterized.
References:
Wąsińska, Małgorzata;Korczewska, Anna;Giurg, Mirosław;Skarzewski, Jacek [Synthetic Communications,2015,vol. 45,# 1,p. 143 - 150] Location in patent:supporting information

576-26-1
344 suppliers
$10.00/25g

2423-71-4
114 suppliers
$7.00/250mg
91-10-1
396 suppliers
$7.00/1g

2423-71-4
114 suppliers
$7.00/250mg
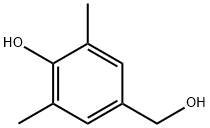
4397-14-2
51 suppliers
$50.00/250 mg

2423-71-4
114 suppliers
$7.00/250mg
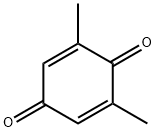
527-61-7
136 suppliers
$30.00/1g
![2-Propenal, 3-[(1,1-dimethylethyl)amino]-2-nitro-](/CAS/20210305/GIF/181294-58-6.gif)