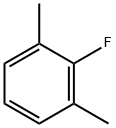
2,6-Dimethylfluorobenzene synthesis
- Product Name:2,6-Dimethylfluorobenzene
- CAS Number:443-88-9
- Molecular formula:C8H9F
- Molecular Weight:124.16
87-62-7

443-88-9
Example 2: General procedure for synthesizing 2-fluoro-1,3-dimethylbenzene from 2,6-dimethylaniline The diazotization of 2,6-dimethylaniline (2.3 g, 0.02 mol) was carried out in an Et3N-3HF system under the same conditions as for 2,4,6-trimethylaniline in Example 1. Upon initial addition of sodium nitrite (2.0 g, 0.03 mol), the color of the reaction mixture gradually changed from yellow to red. A small amount of tar is generated during the reaction, which can be easily separated by solvent extraction. The post-treatment was consistent with the description of 2,4,6-trimethylaniline. The organic layer was extracted from the aqueous phase using diethyl ether (150 mL), and the organic extract was dried over magnesium sulfate and fractionated by solvent to give an orange colored oil. The oily substance was distilled at 141-143 °C and atmospheric pressure to give the colorless liquid 1-fluoro-2,6-dimethylbenzene (2.0 g, 86.3% yield). The distillation process was completed in 1 hour. The 1H NMR (CDCl3) spectrum of the product showed: δ 2.25 (2-CH3 and 6-CH3, d, J = 2.0 Hz, 6H); δ 6.91 (3-H and 5-H, dd, J = 8.3 Hz and J = 6.5 Hz, 2H); and δ 7.01 (4-H, t, J = 8.0 Hz, 1H).19F NMR (CDCl3) spectrum was found to be at δ 122.5 (1-F, ts, J = 6.5 Hz and J = 2.0 Hz) showed signals. Mass spectrometry analysis showed the molecular ion peak at m/z 124 and characteristic fragmentation peaks of 1-fluoro-2,6-dimethylbenzene were observed at m/z 109, 103, 96, 89, 83 and 77.

87-62-7
464 suppliers
$10.00/5 g

443-88-9
192 suppliers
$10.00/5g
Yield:443-88-9 86.3%
Reaction Conditions:
with sodium nitrite in Et3N-3HF;diethyl ether
Steps:
2 Diazotization of 2,6-dimethylaniline in Et3N-3HF
EXAMPLE 2 Diazotization of 2,6-dimethylaniline in Et3N-3HF The diazotization of 2,6-dimethylaniline (2.3 g, 0.02 mol) was performed under the same conditions as described for 2,4,6-trimethylaniline in Example 1. The reaction mixture turned yellow during the initial addition of sodium nitrite (2.0 g, 0.03 mol) and gradually turned red with the increased addition of sodium nitrite. Some tar was formed which was easily extracted with solvent. The work-up was identical to that described for 2,4,6-trimethylaniline. Diethyl ether (150 cm3) was used for the extraction of the organic layer from the aqueous washings. The organic extract was dried over magnesium sulphate. Fractional distillation of the solvent formed an orange oil which was distilled at 141-143° C. ã atmospheric pressure affording 1-fluoro-2,6-dimethylbenzene (2.0 g, 86.3%) as a colourless liquid. The distillation was complete in 1 hour. The 1H n.m.r. spectrum contained signals at δH (CDCl3) 2.25, 2-CH3 and 6-CH3 (d, J=2.0 Hz, 6H); 6.91, 3-H and 5-H (dd, J=8.3 Hz and J=6.5 Hz. 2H) and 7.01, 4-H (t, J=8.0 Hz, 1H). The 19F n.m.r. spectrum had a signal at δF (CDCl3) 122.5, 1-F (ts, J=6.5 Hz and J=2.0 Hz). The mass spectrum produced a molecular ion at m/z 124 and the expected fragmentation pattern for 1-fluoro-2,6-dimethylbenzene at m/z 109, 103, 96, 89, 83 and 77.
References:
Rhodia Limited US6179970, 2001, B2
876-99-3
1 suppliers
inquiry

443-88-9
192 suppliers
$10.00/5g
608-28-6
184 suppliers
$9.00/1g

443-88-9
192 suppliers
$10.00/5g
100379-00-8
300 suppliers
$8.00/1g

443-88-9
192 suppliers
$10.00/5g
108-38-3
383 suppliers
$10.60/50gm:

443-88-9
192 suppliers
$10.00/5g
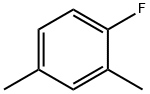
452-65-3
58 suppliers
$17.00/250mg