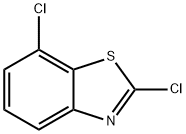
2,7-Dichlorobenzothiazole synthesis
- Product Name:2,7-Dichlorobenzothiazole
- CAS Number:2942-23-6
- Molecular formula:C7H3Cl2NS
- Molecular Weight:204.08
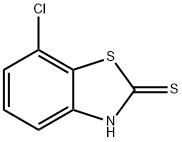
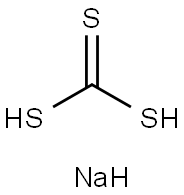
1849-73-6

2942-23-6
General procedure for the synthesis of 2,7-dichlorobenzo[d]thiazole from 7-chlorobenzo[d]thiazole-2(3H)-thione: General method: a mixture of 7-chlorobenzo[d]thiazole-2(3H)-thione (>1 g, 1 eq.) and sulfuryl chloride (10 eq.) was stirred at 20-25 °C for 15 min. Subsequently, water (2 eq.) was added and stirring was continued for 3 hours at 20-25 °C. During the reaction, the sample was removed, quenched with acetonitrile/water (2:1) and analyzed by high performance liquid chromatography (HPLC). Upon completion of the reaction, the mixture was diluted with acetonitrile (5 v/v) and the reaction was quenched by slowly adding water (20 v/v). The product precipitated from the aqueous solution and the solid was collected and washed with distilled water. Finally, the solid was dried under vacuum to give pure 2,7-dichlorobenzo[d]thiazole. For the liquid product 2-chlorobenzo[d]thiazole (13), the reaction mixture was extracted with ethyl acetate and the organic layer was dried and concentrated to give an oily product.

1849-73-6
68 suppliers
$9.00/100mg

2942-23-6
79 suppliers
$25.00/100mg
Yield: 94%
Reaction Conditions:
Stage #1:2-mercapto-7-chlorobenzothiazole with sulfuryl dichloride at 20 - 25; for 0.25 h;Inert atmosphere;
Stage #2: with water at 20 - 25; for 3 h;Inert atmosphere;
Steps:
Chlorination Using Sulfuryl Chloride/Water; General Procedure
General procedure: A mixture of the 2-mercaptobenzo[d]thiazole (>1 g, 1 equiv) and sul-furyl chloride (10 equiv) was stirred at 20-25 °C for 15 min. Next, H 2 O(2 equiv) was added and the mixture was stirred at 20-25 °C for anadditional 3 h. A sample was taken, quenched with MeCN/H 2 O (2:1)and analyzed by HPLC. After completion of the reaction, the mixturewas diluted with MeCN (5 volumes) and slowly quenched with H 2 O(20 volumes). The product precipitated from the aqueous solution.The solid was collected and washed with H 2 O. Drying under vacuumafforded the pure product. In the case of the liquid product 2-chloro-benzo[d]thiazole (13), the reaction mixture was extracted withEtOAc. The organic layer was then dried and concentrated to affordthe product as an oil.
References:
Wimmer, Laurin;Parmentier, Michael;Riss, Bernard;Kapferer, Tobias;Ye, Chao;Li, Lei;Kim, Hongyong;Li, Jialiang [Synthesis,2018,vol. 50,# 10,p. 2027 - 2032]
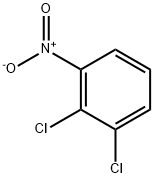
3209-22-1
20 suppliers
$10.00/1g

2942-23-6
79 suppliers
$25.00/100mg
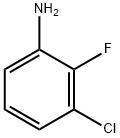
2106-04-9
258 suppliers
$6.00/10g

2942-23-6
79 suppliers
$25.00/100mg
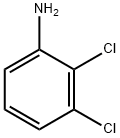
608-27-5
231 suppliers
$13.00/5g

2942-23-6
79 suppliers
$25.00/100mg