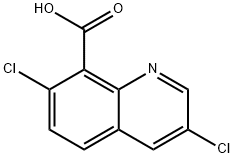
Quinclorac synthesis
- Product Name:Quinclorac
- CAS Number:84087-01-4
- Molecular formula:C10H5Cl2NO2
- Molecular Weight:242.06
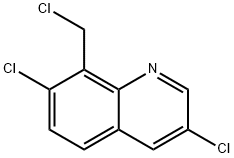
84086-96-4
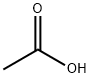
64-19-7

84087-01-4
A high-pressure reaction solution consisting of 80.0 g of 3,7-dichloro-8-chloromethylquinoline, 300.0 g of glacial acetic acid, 5.7 g of cobalt acetate tetrahydrate, 1.8 g of manganese acetate tetrahydrate, and 4.9 g of 40% hydrogen bromide was added to a 0.5 L titanium reactor. Oxygen was pressurized to 1.0 MPa and the reaction solution was heated under stirring to 150°C, at which point the pressure was increased to 1.6 MPa. The reaction was terminated after 4.1 hours when the reaction system no longer consumed oxygen. The reaction mixture was then cooled to room temperature and the solid product was isolated and dried to afford 75.0 g of 3,7-dichloroquinoline-8-carboxylic acid as crude product at 86.8%. 75.0 g of the crude product was added to 200.0 g of anhydrous ethanol, stirred and heated to reflux and kept for 2 hours. After cooling to below 10°C, it was filtered, the filtrate was distilled to recover ethanol, and the filter cake was dried to obtain 63.8 g of 3,7-dichloroquinoline-8-carboxylic acid as pure product with 97.9% content and 96.0% yield.

84086-96-4
3 suppliers
inquiry

64-19-7
1626 suppliers
$10.00/25ML

84087-01-4
292 suppliers
$40.00/10g
Yield:84087-01-4 96.2%
Reaction Conditions:
with hydrogen bromide;manganese (II) acetate tetrahydrate;cobalt(II) diacetate tetrahydrate at 150; under 7500.75 - 12001.2 Torr; for 4.2 h;Concentration;
Steps:
1 preparation of quinchlorac acid
To the volume of 0.5L titanium reactor by adding high-pressure reaction solution,Oxygen into the pressure to l.OMpa,While stirring, the reaction solution was heated to 150 ° C,The pressure rose to 1.6 MPa. The composition of the reaction mixture (i.e., the reaction mixture) was 80.0 g of 7-chloro-8-methylquinoline chloride and 300. Og of acetic acid,5.7 g of cobalt acetate tetrahydrate,1.8 g of manganese acetate tetrahydrate,4.9 g of 40% hydrogen bromide. Reaction 4. lh,The reaction system no longer consumes oxygen,The reaction was terminated. And then discharged to room temperature,The solid product was isolated,dry.getDichloroquinoline acid dry goods75. Og,Content of 86.8%.[0221]75. OgDichloroquinoline acid added to the crude200. Og absolute ethanol,Stirring heated to reflux,Insulation 2h,Cooled to below 10 ° C,filter,Filtrate distillation recovery of ethanol applied,Filter cake drying,TooQuincloracfineProducts63.8 g,Content 97.9%The yield was 96.0%.
References:
CN103420909,2016,B Location in patent:Paragraph 0019; 0020

84086-96-4
3 suppliers
inquiry

84087-01-4
292 suppliers
$40.00/10g
88347-01-7
2 suppliers
inquiry

84087-01-4
292 suppliers
$40.00/10g
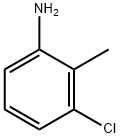
87-60-5
445 suppliers
$8.00/25g

84087-01-4
292 suppliers
$40.00/10g
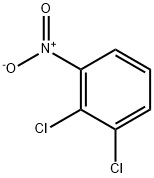
3209-22-1
20 suppliers
$10.00/1g

84087-01-4
292 suppliers
$40.00/10g