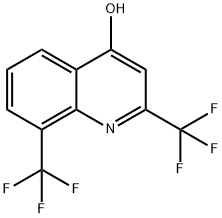
2,8-Bis(trifluoromethyl)-4-quinolinol synthesis
- Product Name:2,8-Bis(trifluoromethyl)-4-quinolinol
- CAS Number:35853-41-9
- Molecular formula:C11H5F6NO
- Molecular Weight:281.15
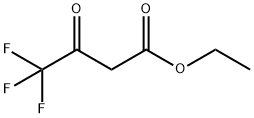
372-31-6
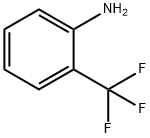
88-17-5

35853-41-9
2-(Trifluoromethyl)aniline (0.54 mL, 4.3 mmol) and ethyl 4,4,4-trifluoro-3-oxobutanoate (0.62 mL, 4.3 mmol) were mixed in a round-bottomed flask fitted with a large oval stir bar. Subsequently, polyphosphoric acid (4.0 g) was added to the reaction system. The reaction mixture was stirred at 120 °C for 3 h under nitrogen protection. Upon completion of the reaction, the reaction was quenched with ice water (50 mL) to give a golden-brown sticky substance. Extraction was carried out with dichloromethane (2 x 20 mL) and the organic layers were combined and dried over anhydrous magnesium sulfate. After filtration, the organic phase was concentrated under reduced pressure to give a beige solid product that did not require further purification (0.27 g, 23% yield).

372-31-6
488 suppliers
$5.00/10g

88-17-5
372 suppliers
$14.00/25g

35853-41-9
217 suppliers
$15.00/1g
Yield:35853-41-9 91%
Reaction Conditions:
with polyphosphoric acid at 150; for 3 h;
Steps:
1 4.1.1. 2,8-bis(trifluoromethyl)quinolin-4-ol (19)
To a solution of polyphosphoric acid (637.7 mmol) in 2-trifluoromethylaniline (77.6 mmol) was added ethyl 4,4,4-trifluoroacetoacetate(77.7 mmol). The reaction mixture was stirred for3 h at 150 °C. Completion of the reaction was monitored by TLC. After,the reaction mixture was poured into iced distilled water slowly withvigorous stirring to form yellow precipitate. The precipitate was vacuumfiltered and washed with cold distilled water to give yellow solidin 91% yield. m.p 128-130 °C; 1H NMR (400 MHZ; DMSO-d6, ppm): δ7.30 (s, 1H, H-3); 7.80 (t, 1H, H-6; J=7.9 Hz); 8.28 (d, 1H, H-5;J=7.2 Hz); 8.52 (d, 1H, H-7, J=8.2 Hz); 13C NMR (100 MHz, DMSO-d6, ppm): δ 101.0 (C-3); 119.9 (C-4a); 121.9 (C-6); 122.6 (q, C-9,J=273 Hz); 125.3 (q, C-10, J=276 Hz); 127.3 (C-7); 130.0 (q, C-8,J=58 Hz); 144.5 (C-5); 148.4 (q, C-2, J=67 Hz); 163.3 (C-4); 19FNMR (376 MHZ; DMSO-d6, ppm): δ -58.98 (s, 3F; F-10); -67.03 (s,3F; F-9).
References:
da Silva, Renata M.R.J.;Gandi, Marilia O.;Mendon?a, Jorge S.;Carvalho, Alcione S.;Coutinho, Julia Penna;Aguiar, Anna C.C.;Krettli, Antoniana U.;Boechat, Nubia [Bioorganic and Medicinal Chemistry,2019,vol. 27,# 6,p. 1002 - 1008]