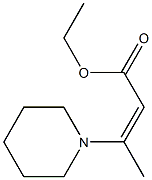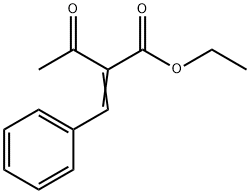
2-Acetyl-3-phenylacrylic acid ethyl ester synthesis
- Product Name:2-Acetyl-3-phenylacrylic acid ethyl ester
- CAS Number:620-80-4
- Molecular formula:C13H14O3
- Molecular Weight:218.25

141-97-9

100-52-7

620-80-4
To an anhydrous DMSO solution containing L-proline (30 mol%) was added benzaldehyde (1 eq.). The reaction mixture was stirred at room temperature for 8-10 minutes, followed by the slow addition of ethyl acetoacetate (1.2 eq.) at the same temperature. The reaction system was warmed up to 80 °C with continuous stirring for 6-8 hours. Upon completion of the reaction, ethyl acetate (10 mL) was added to the mixture and stirred for several minutes before quenching the reaction with cold water. The organic and aqueous phases were separated and the aqueous phase was extracted with ethyl acetate (3 x 20 mL). The organic phases were combined, washed with saturated saline and dried with anhydrous sodium sulfate. After concentration under reduced pressure to remove the solvent, the crude product was purified by column chromatography on silica gel (60-120 mesh), the eluent being a hexane solution of 5% ethyl acetate.
Yield:620-80-4 97%
Reaction Conditions:
with G/MFSiO2Cu(proline)2 at 20; for 5 h;Green chemistry;
Steps:
Aldol reactions catalyzed by G/MFSiO2Cu(proline)2
General procedure: A mixture of benzaldehyde and cyclohexanone at molar ratio of 1:2 mmol and nanocatalyst(0.1 g) was stirred at 40-60 °C; the progress of the condensation reactionwas monitored continuously by thin-layer chromatography (TLC). Ethyl acetate(10 mL) was then added to the reaction mixture, and the nanocatalyst was magneticallyseparated and washed with ethyl acetate and dried for reuse in a new aldolreaction. The remaining liquid was washed with saturated aqueous sodium chloridesolution (5 mL) and water (10 mL). The organic layer, which contains the productof the aldol reaction, was separated and dried over anhydrous Na2SO4,followedby evaporation of the solvent at room temperature. The remaining residue was thenrecrystallized in ethanol to give pure aldol product with 98 % yield. The yields inthis and other similar reactions were measured by GC analysis. Identification of theproducts for a number of selected reactions between cyclohexanone and benzaldehydederivatives was also performed by 1H NMR and GC-mass spectrometry (MS)techniques. The spectral data of selected products are given below.
References:
Kooti;Kooshki;Nasiri [Research on Chemical Intermediates,2019,vol. 45,# 5,p. 2641 - 2656]

141-97-9
826 suppliers
$5.00/25g

620-80-4
55 suppliers
$101.00/1g