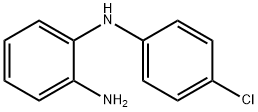
N1-(4-Chlorophenyl)benzene-1,2-diamine synthesis
- Product Name:N1-(4-Chlorophenyl)benzene-1,2-diamine
- CAS Number:68817-71-0
- Molecular formula:C12H11ClN2
- Molecular Weight:218.68
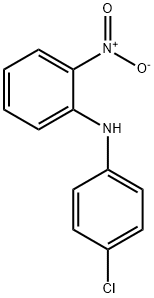
23008-56-2
103 suppliers
$10.00/250mg

68817-71-0
117 suppliers
$45.00/1g
Yield:68817-71-0 94%
Reaction Conditions:
with Aminoiminomethanesulfinic acid;sodium hydroxide in ethanol;lithium hydroxide monohydrate at 50; for 1.5 h;Reagent/catalyst;Solvent;Temperature;
Steps:
General procedure for the synthesis of 4 in Table 3
General procedure: A mixture of 3 (10 mmol) and NaOH (4.0 g, 100 mmol) was prepared in EtOH (5 mL) and H2O (15 mL). TDO (5.4 g, 50 mmol) was added in batches within 15 minutes to the mixture followed by stirring at 50 °C. After completion of the reaction as indicated by TLC, cooled to room temperature, the precipitated solid was filtered out and washed with water to obtain compound 4.
References:
Cui, Jian-Lan;Wang, Ning;Wang, Xiao;Yu, Si-Yuan;Zhong, Cong-Shan [Tetrahedron Letters,2020,vol. 61,# 11] Location in patent:supporting information
![2-Phenazinamine, 5-(4-chlorophenyl)-3-[(4-chlorophenyl)imino]-3,5-dihydro-](/CAS/20210305/GIF/96762-04-8.gif)
96762-04-8
1 suppliers
inquiry

68817-71-0
117 suppliers
$45.00/1g
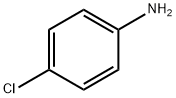
106-47-8
483 suppliers
$5.00/5G

68817-71-0
117 suppliers
$45.00/1g
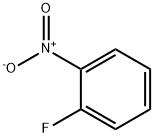
1493-27-2
511 suppliers
$5.00/10g

68817-71-0
117 suppliers
$45.00/1g
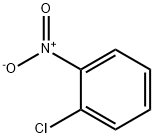
88-73-3
49 suppliers
$14.00/25g

68817-71-0
117 suppliers
$45.00/1g