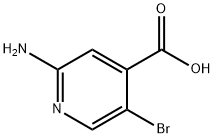
2-Amino-5-bromoisonicotinic acid synthesis
- Product Name:2-Amino-5-bromoisonicotinic acid
- CAS Number:1000339-23-0
- Molecular formula:C6H5BrN2O2
- Molecular Weight:217.02
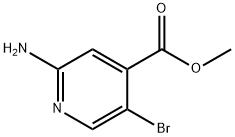
882499-87-8

1000339-23-0
General procedure for the synthesis of 2-amino-5-bromo-4-carboxylic acid pyridine from 2-amino-5-bromoisonicotinic acid methyl ester: 2-amino-5-bromoisonicotinic acid methyl ester (10.0 g, 43.28 mmol) and lithium hydroxide (LiOH, 5.46 g, 129.8 mmol) were reacted with 2-amino-5-bromo-4-carboxylic acid methyl ester (10.0 g, 43.28 mmol) and 2-amino-5-bromoisonicotinic acid methyl ester (10.0 g, 43.28 mmol) and lithium hydroxide (LiOH, 5.46 g, 129.8 mmol) in a water (100 mL) in a solvent mixture of tetrahydrofuran (THF, 400 mL), methanol (MeOH, 200 mL), and water (100 mL), and the reaction was stirred at 70 °C for 2 h under nitrogen (N2) protection. Upon completion of the reaction, the reaction mixture was cooled to 0 °C and the pH was adjusted to 6 by dropwise addition of concentrated aqueous hydrochloric acid (HCl).Subsequently, the resulting suspension was filtered and the solid was dried under vacuum to afford pyridine 2-amino-5-bromo-4-carboxylic acid (7.8 g, 83% yield). The product was analyzed by liquid chromatography-mass spectrometry (LC-MS, ESI) and showed m/z 211 [M + H]+.

882499-87-8
148 suppliers
$47.00/1g

1000339-23-0
86 suppliers
$8.00/250mg
Yield: 83%
Reaction Conditions:
Stage #1:methyl 2-amino-5-bromopyridine-4-carboxylate with water;lithium hydroxide in tetrahydrofuran;methanol at 70; for 2 h;Inert atmosphere;
Stage #2: with hydrogenchloride in tetrahydrofuran;methanol;water; pH=6 at 0;
Steps:
14.1
A mixture of compound 14-1 (10.0 g, 43.28 mmol) and LiOH (5.46 g, 129.8 mmol) in THF (400 mL), MeOH (200 mL) and water (100 mL) was stirred at 70 °C for 2 hrs under an atmosphere of N2. Subsequently, the reaction mixture was cooled to 0 °C and adjusted its pH value to 6 by adding coned, aq. HC1. The resulting suspension was filtered and the solid was dried in vacuo to give compound 14-2 (7.8 g, 83% yield). LC-MS (ESI): m/z 211 [M+H]+.
References:
PRESIDIO PHARMACEUTICALS, INC.;ZHONG, Min;LI, Leping WO2012/58125, 2012, A1 Location in patent:Page/Page column 145