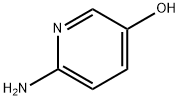
2-Amino-5-hydroxypyridine synthesis
- Product Name:2-Amino-5-hydroxypyridine
- CAS Number:55717-46-9
- Molecular formula:C5H6N2O
- Molecular Weight:110.11
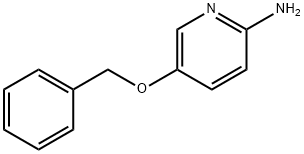
96166-00-6

55717-46-9
The general procedure for the synthesis of 2-amino-5-hydroxypyridine from 5-(benzyloxy)pyridin-2-amine was as follows: 2-amino-5-benzyloxypyridine, ethanol (217 g) and a toluene solution (331 g) of 10% Pd/C (2.28 g, 53% moisture content) were added to an autoclave and the reaction was carried out at 25 °C. During the reaction, hydrogen was introduced into the autoclave, maintaining an absolute pressure of 0.2 MPa, and the reaction lasted for several hours. Upon completion of the reaction, the Pd/C catalyst was removed by filtration and the residue was washed with ethanol (76 g). The filtrate was dried under reduced pressure to give 2-amino-5-hydroxypyridine (25.6 g, 0.23 mol) in 92% yield.

96166-00-6
81 suppliers
$85.00/1g

55717-46-9
179 suppliers
$45.00/10mg
Yield:55717-46-9 92%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in ethanol;toluene at 25; under 1500.15 Torr; for 4 h;Autoclave;
Steps:
4
A toluene solution (331 g) of the obtained 2-amino-5-benzyloxypyridine,Ethanol (217 g) and 10% Pd / C (2.28 g, moisture content 53%) were charged in an autoclave and reacted at 25 ° C. for 4 hours while being introduced at 0.2 MPa (absolute pressure) in a hydrogen atmosphere.After completion of the reaction, Pd / C was filtered and the residue was washed with ethanol (76 g).The filtrate was dried under reduced pressure to obtain 2-amino-5-hydroxypyridine (25.6 g, 0.23 mol) (yield 92%).
References:
KOEI CHEMICAL COMPANY LIMITED;TAKAHASHI, SATOSHI;NIHEI, YURI JP2017/48131, 2017, A Location in patent:Paragraph 0124
10167-97-2
226 suppliers
$18.00/1g

55717-46-9
179 suppliers
$45.00/10mg

69634-17-9
3 suppliers
inquiry

55717-46-9
179 suppliers
$45.00/10mg
109-00-2
610 suppliers
$10.00/10g

55717-46-9
179 suppliers
$45.00/10mg
35771-41-6
3 suppliers
$144.00/50mg

55717-46-9
179 suppliers
$45.00/10mg