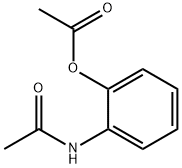
2-AMINOPHENOL-N,O-DIACETATE synthesis
- Product Name:2-AMINOPHENOL-N,O-DIACETATE
- CAS Number:5467-64-1
- Molecular formula:C10H11NO3
- Molecular Weight:193.2
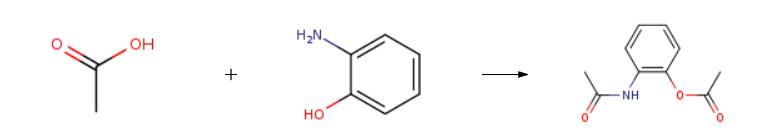

108-24-7
0 suppliers
$14.00/250ML
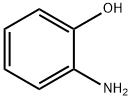
95-55-6
557 suppliers
$5.00/5G

5467-64-1
19 suppliers
$28.60/250mg
Yield:5467-64-1 99.1%
Reaction Conditions:
with sulfuric acid in methanol; for 4 h;Reflux;Large scale;Concentration;
Steps:
2.1 preparation of 2-acetamidophenyl acetate
Take 2-aminophenol (110kg),Acetic anhydride (225 kg),Methanol 410kg,11 liters of concentrated sulfuric acid,Reflux reaction 4h. After the reaction,Methanol recovery under reduced pressure,Cool to room temperature,To the reaction system was added saturated sodium bicarbonate solution was adjusted to pH9-10,410kg ethyl acetate extraction,Washed to neutral,Dried over anhydrous sodium sulfate,filter,The solvent was recovered under reduced pressure,Had a light yellow solid 191.5kg,Yield 99.1%.
References:
CN106995366,2017,A Location in patent:Paragraph 0016; 0018

64-19-7
1627 suppliers
$10.00/25ML

95-55-6
557 suppliers
$5.00/5G

5467-64-1
19 suppliers
$28.60/250mg

64-19-7
1627 suppliers
$10.00/25ML
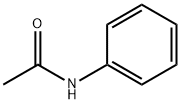
103-84-4
355 suppliers
$12.00/100g

5467-64-1
19 suppliers
$28.60/250mg

108-24-7
0 suppliers
$14.00/250ML
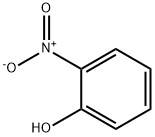
88-75-5
37 suppliers
$15.00/5mg

5467-64-1
19 suppliers
$28.60/250mg

75-36-5
589 suppliers
$17.92/100G
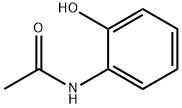
614-80-2
319 suppliers
$5.00/10g

5467-64-1
19 suppliers
$28.60/250mg