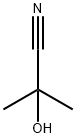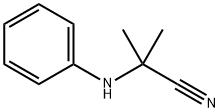
2-anilino-2-methylpropiononitrile synthesis
- Product Name:2-anilino-2-methylpropiononitrile
- CAS Number:2182-38-9
- Molecular formula:C10H12N2
- Molecular Weight:160.22
Yield: 94%
Reaction Conditions:
for 20 h;Heating / reflux;
Steps:
6.1
2-methyl-2-phenylaminopropanenitrile, 6a A mixture of aminobenzene (0.931 g, 10 mmol) and acetone cyanohydrin (2 ml) was heated to reflux and stirred for 20 h. After being cold to room temperature, the reaction mixture was poured into ethyl acetate (40 ml) and washed with cold water (2 x 30 ml). The organic layer was dried over MgSO4, concentrated under vacuum to dryness to yield 2-methyl-2-phenylaminopropanenitrile, 6a (1.5 Ig, 9.4 mmol, 94%) as slurry brown liquid.
References:
THE REGENTS OF THE UNIVERSITY OF CALIFORNIA WO2006/124118, 2006, A1 Location in patent:Page/Page column 26
7677-24-9
394 suppliers
$19.00/5g

62-53-3
689 suppliers
$10.00/1g

67-64-1
0 suppliers
$17.30/10ml

2182-38-9
16 suppliers
inquiry
631-57-2
72 suppliers
$31.80/1g

62-53-3
689 suppliers
$10.00/1g

67-64-1
0 suppliers
$17.30/10ml

2182-38-9
16 suppliers
inquiry
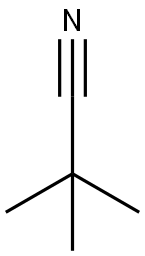
630-18-2
197 suppliers
$10.00/1g

62-53-3
689 suppliers
$10.00/1g

67-64-1
0 suppliers
$17.30/10ml

2182-38-9
16 suppliers
inquiry

62-53-3
689 suppliers
$10.00/1g

2182-38-9
16 suppliers
inquiry