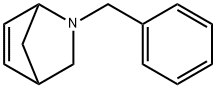
2-BENZYL-2-AZABICYCLO[2.2.1]HEPT-5-ENE synthesis
- Product Name:2-BENZYL-2-AZABICYCLO[2.2.1]HEPT-5-ENE
- CAS Number:112375-05-0
- Molecular formula:C13H15N
- Molecular Weight:185.26

50-00-0
841 suppliers
$10.00/25g
39110-74-2
31 suppliers
inquiry
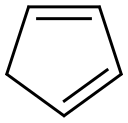
542-92-7
92 suppliers
inquiry
![2-BENZYL-2-AZABICYCLO[2.2.1]HEPT-5-ENE](/CAS/GIF/112375-05-0.gif)
112375-05-0
41 suppliers
$60.00/100mg
Yield:-
Reaction Conditions:
in water at 20; for 3 h;
Steps:
4.1.1. Synthesis of azanorbornenes
Azanorbornenes were produced as described in our previous work[11].To obtainN-benzyl-2-azanorbornene-5 benzylamine hydrochloride (0.20mol, 28.8g) was dissolved in water (75ml) and 37% aqueous formaldehyde (0.34mol, 26ml) was added to the solution. After that freshly prepared cyclopentadiene (0.51mol, 33.67g) was added drop wise under vigorously stirring and the reactive mixture was stirred for 3h at room temperature. The reaction was washed twice with ether:hexane mixture (1:1). The aqueous phase was made basic with solid potassium hydroxide. The product was isolated by extraction with ether.To obtainN-allyl-2-azanorbornene-5 the reaction flask was cooled in an ice bath and was loaded consecutively with water (75ml), concentrated HCl (0.20mol), allylamine (0.2mol, 11.4g), 37% aqueous formaldehyde (0.34mol, 26ml) and freshly prepared cyclopentadiene (0.51mol, 33.67g). The reactive mixture was stirred for 3h at room temperature. The reaction was washed twice with ether:hexane mixture (1:1). The aqueous phase was made basic with solid potassium hydroxide. The product was isolated by extraction with ether.To obtainN-(2-azanorbornene-5-en)methylacetate glycine methyl ester hydrochloride (0.10mol, 12.6g) was dissolved in water (50ml) and 37% aqueous formaldehyde (0.20mol, 15ml) was added to the solution. After that cyclopentadiene (0.30mol, 19.8g) was added drop wise under vigorously stirring and the reactive mixture was stirred for 3h at room temperature. The reaction was washed twice with ether:hexane mixture (1:1). The aqueous phase was made basic with solid potassium hydroxide. The product was isolated by extraction with ether.The yields of BANB, AANB and ANBM were 82, 74 and 70% from the theory, respectively. The fractions ofN-benzyl-2-azanorbornene-5 withTb=99-102°C (66.6Pa),nD20=1.5489;N-allyl-2-azanorbornene-5 withTb=51-53°C (133.3Pa),nD20=1.4880;N-(2-azanorbornene-5-en)methylacetate withTb=80-82°C (160.0Pa),nD20=1.4830 were used for copolymerization. The structures of the compounds were confirmed by13C NMR spectra (Table1).
References:
Gorbunova, Marina;Anikina, Lada [European Journal of Medicinal Chemistry,2013,vol. 63,p. 655 - 661]