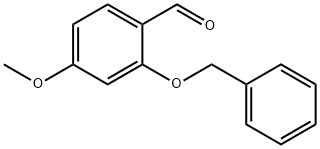
2-(Benzyloxy)-4-methoxybenzenecarbaldehyde synthesis
- Product Name:2-(Benzyloxy)-4-methoxybenzenecarbaldehyde
- CAS Number:32884-23-4
- Molecular formula:C15H14O3
- Molecular Weight:242.27
673-22-3

100-39-0

32884-23-4
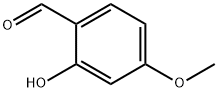
General procedure for the synthesis of 2-(benzyloxy)-4-methoxybenzaldehyde from 2-hydroxy-4-methoxybenzaldehyde and benzyl bromide: 2-hydroxy-4-methoxybenzaldehyde (1 g, 6.57 mmol) was dissolved in ethanol (100 mL), K2CO3 and benzyl bromide were added. The reaction mixture was heated and stirred at 50 °C for two days. After completion of the reaction, the reaction solution was concentrated and the residue was dissolved in diethyl ether. The ether solution was washed sequentially with brine, 5% NaOH aqueous solution and water, and then dried with anhydrous Na2SO4. After filtration, the solvent was evaporated to give 2-(benzyloxy)-4-methoxybenzaldehyde (1.5 g, 95% yield): LC-MS [M + H]+ = 243.02.
Yield:32884-23-4 95%
Reaction Conditions:
with potassium carbonate in ethanol at 50; for 48 h;
Steps:
4.a.a
2-(Benzyloxy)-4-methoxybenzaldehyde[0150] K2CO3 and benzyl bromide were added to a solution of 2-hydroxy-4- methoxy benzaldehyde (1 g, 6.57 mmol) in ethanol (100 mL), and the mixture was then heated for two days at 50 0C. The solution was concentrated and the residue was dissolved in-55-SDI-10975vl Attorney Docket No. 12560-046-228diethyl ether. The ether solution was washed with brine, 5% aqueous NaOH, water, dried (Na2SO4) and evaporated to give the 2-benzyloxy-4-methoxybenzaldehyde (1.5 g, 95%): LC-MS [M+H]+= 243.02.
References:
WO2010/111353,2010,A1 Location in patent:Page/Page column 55-56

673-22-3
497 suppliers
$5.00/1g

100-44-7
659 suppliers
$13.50/250G

32884-23-4
42 suppliers
$70.00/1g