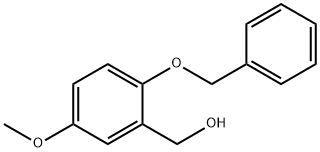
(2-Benzyloxy-5-methoxy-phenyl)-methanol synthesis
- Product Name:(2-Benzyloxy-5-methoxy-phenyl)-methanol
- CAS Number:214072-73-8
- Molecular formula:C15H16O3
- Molecular Weight:244.29
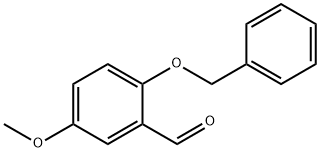
56979-57-8
11 suppliers
inquiry

214072-73-8
7 suppliers
inquiry
Yield:214072-73-8 98%
Reaction Conditions:
with sodium tetrahydridoborate in methanol at 0 - 20; for 1.33 h;
Steps:
Compound 3
Compound 2 (22.67 g, 93.68 mmol) was dissolved in MeOH (150 mL) in a round-bottomed flask and the solution was cooled in an ice-bath to produce a fine suspension. NaBH4 (4.25 g, 112.43 mmol)was carefully added and allowed to stand for 20 min; the solution was allowed to warm up to room temperature and stirred for 1 h. TLC analysis indicated when the reaction was complete. After removal of MeOH, the reaction mixture was poured into water (80 mL), and extracted with EtOAc (80 mL) for three times. The combined organic layers were washed successively with a saturated NH4Cl solution, water, and brine, and then dried over anhydrous MgSO4. The solvent was removed in vacuo to afford 3(22.40 g, 98%) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.34-7.25 (m, 5H), 6.90(d, J = 3.0 Hz, 1H), 6.78 (d, J = 8.5 Hz, 1H), 6.69 (dd, J = 8.5, 3.0 Hz, 1H), 4.95 (s, 2H), 4.64(s, 2H), 3.67 (s, 3H), 2.81 (br s, 1H, OH); 13C NMR (400 MHz, CDCl3) δ 153.70,149.96, 137.01, 130.86, 128.33, 128.33, 127.66, 127.03, 127.03, 113.94, 112.70,112.57, 70.42, 60.66, 55.30; MS (ESI) m/z 267 (M+Na)+.
References:
Li, Rui-Juan;Zhao, Yu;Tokuda, Harukuni;Yang, Xiao-Ming;Wang, Yue-Hu;Shi, Qian;Morris-Natschke, Susan L.;Lou, Hong-Xiang;Lee, Kuo-Hsiung [Tetrahedron Letters,2014,vol. 55,# 47,p. 6500 - 6503] Location in patent:supporting information

67-56-1
790 suppliers
$9.00/25ml
121124-94-5
73 suppliers
$24.00/250mg

214072-73-8
7 suppliers
inquiry
150-76-5
817 suppliers
$5.00/5G

214072-73-8
7 suppliers
inquiry
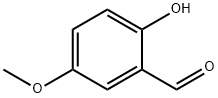
672-13-9
277 suppliers
$39.00/5g

214072-73-8
7 suppliers
inquiry
