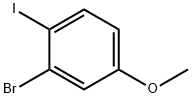
2-bromo-1-iodo-4-methoxybenzene synthesis
- Product Name:2-bromo-1-iodo-4-methoxybenzene
- CAS Number:466639-53-2
- Molecular formula:C7H6BrIO
- Molecular Weight:312.93
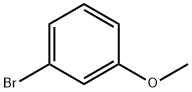
2398-37-0

466639-53-2
General procedure for the synthesis of 2-bromo-1-iodo-4-methoxybenzene using 3-bromoanisole as starting material: 3-bromoanisole (10 g, 53.5 mmol), mercuric oxide (HgO, 8.8 g, 40.6 mmol) and acetic anhydride (Ac2O, 1 mL) were dissolved in dichloromethane (CH2Cl2, 100 mL) under stirring conditions, and refluxed for 30 minutes. Subsequently, iodine (I2, 17.6 g, 69.5 mmol) was added in six 12-hour intervals, and refluxing was continued for 12 hours after each addition. Upon completion of the reaction, the reaction mixture was filtered through a diatomaceous earth pad and the filtrate was washed with saturated sodium thiosulfate (Na2S2O3) solution. The aqueous layer was extracted with dichloromethane (3 x 10 mL), the organic layers were combined and dried over anhydrous magnesium sulfate (MgSO4) and subsequently concentrated to dryness under reduced pressure. The crude product was purified by fast column chromatography (with cyclohexane as eluent) to afford the target compound 2-bromo-1-iodo-4-methoxybenzene as a colorless oil (94% yield). The product was characterized by 1H NMR (300 MHz, CDCl3): δ 7.68 (d, J = 8.8 Hz, 1H), 7.19 (d, J = 2.8 Hz, 1H), 6.59 (dd, J = 2.8 Hz, J = 8.8 Hz, 1H), 3.77 (s, 3H).
32338-02-6
138 suppliers
$14.00/1g

466639-53-2
107 suppliers
$9.00/250mg
Yield: 92%
Reaction Conditions:
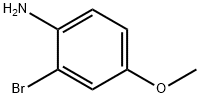
Stage #1:2-bromo-4-methoxyaniline with hydrogenchloride;sodium nitrite in water;acetonitrile at 0 - 5; for 0.5 h;Sandmeyer Reaction;
Stage #2: with potassium iodide in water;acetonitrile at 20;Sandmeyer Reaction;
Steps:
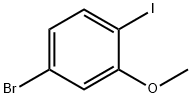
1) 2-bromoiodobenzene (2a) as general procedure
General procedure: To a solution of 2-bromoaniline (5 g, 29 mmol) dissolved in CH3CN (80 mL) was added aq. HCl (15 mL conc. HCl in 50 mL water), then the mixture was cooled to 0 °C, and it was added a solution of NaNO2 (2.4 g, 34.87 mmol) in water (50 mL). After addition, the reaction was kept at the temperature lower than 5 °C for 30 min and it was added a solution of (7.23 g, 43.59 mmol) in water (50 mL). After addition, the reaction was kept at room temperature overnight, poured into water (300 mL) and extracted with CH2Cl2.The organic phase was dried over MgSO4. After workup, the brown oily product was distilled to afford a pale-yellow.
References:
Lv, Jun;Liu, Qiancai;Tang, Jie;Perdih, Franc;Kranjc, Kristof [Tetrahedron Letters,2012,vol. 53,# 39,p. 5248 - 5252] Location in patent:supporting information

2398-37-0
586 suppliers
$8.00/10g

466639-53-2
107 suppliers
$9.00/250mg

2398-37-0
586 suppliers
$8.00/10g

466639-53-2
107 suppliers
$9.00/250mg
791642-68-7
119 suppliers
$10.00/250mg
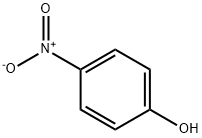
100-02-7
61 suppliers
$11.00/5G

466639-53-2
107 suppliers
$9.00/250mg
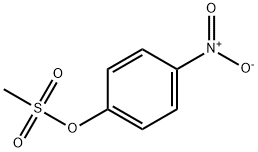
20455-07-6
4 suppliers
inquiry

466639-53-2
107 suppliers
$9.00/250mg