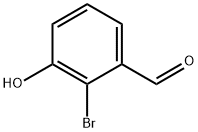
2-Bromo-3-hydroxybenzaldehyde synthesis
- Product Name:2-Bromo-3-hydroxybenzaldehyde
- CAS Number:196081-71-7
- Molecular formula:C7H5BrO2
- Molecular Weight:201.02

100-83-4

196081-71-7
The general procedure for the synthesis of 2-bromo-3-hydroxybenzaldehyde from m-hydroxybenzaldehyde was as follows: 3-hydroxybenzaldehyde (5 g, 0.04 mol), iron powder (172 mg, 3 mmol), and sodium acetate (6.72 g, 0.08 mol) were suspended in acetic acid (40 mL), warmed until a clarified solution was formed, and subsequently cooled to room temperature. A glacial acetic acid solution of bromine (10 mL) was slowly added dropwise to the mixture over 15 minutes. After the dropwise addition was completed, stirring of the reaction mixture was continued for 2 hours. Upon completion of the reaction, the mixture was poured into ice water and extracted with dichloromethane (3 x 50 mL). The organic phases were combined, dried over anhydrous Na2SO4 and concentrated. The residue was recrystallized by dichloromethane to afford the target product 2-bromo-3-hydroxybenzaldehyde (2.3 g, 28% yield). The product was characterized by 1H NMR (400 MHz, DMSO-d6): δ 10.30 (s, 1H), 7.54-7.51 (m, 1H), 7.39-7.35 (m, 1H), 7.31-7.27 (m, 1H), 5.90 (s, 1H).

100-83-4
648 suppliers
$5.00/10g

196081-71-7
145 suppliers
$9.00/1g
Yield:196081-71-7 44%
Reaction Conditions:
with bromine;sodium acetate;iron;acetic acid at 20; for 5 h;Inert atmosphere;
Steps:
To a stirred suspension of 3-hydroxybenzaldehyde (4.88 g, 40.0 mmol), AcONa (6.56 g, 80.0 mmol),and iron powder (167 mg, 3.00 mmol, purchased from Kanto Chemical Co., Inc. Japan) in AcOH (36mL) was added Br2 (7.35 g, 46.0 mmol) in AcOH (8 mL) dropwise at room temperature. Afterbeing stirred at room temperature for 5 h, the reaction mixture was filtered through Celite with aid ofethyl acetate and the solvent was removed under reduced pressure. The residue was dissolved inethyl acetate and washed successively with aqueous saturated Na2S2O3 solution and brine, dried overNa2SO4, and concentrated in vacuo to give a crude yellowish solid, which was chromatographed onsilica gel (hexane-ethyl acetate) to afford 2-bromo-3-hydroxybenzaldehyde (3.58 g, 44%) as a whitesolid
References:
Hata, Takeshi;Hayashi, Yoshiki;Hasegawa, Yuki;Iwai, Masaaki;Ishii,, Ayumi;Hasegawa, Miki;Shigeta, Masayuki;Urabe, Hirokazu [Chemistry Letters,2019,vol. 48,# 7,p. 662 - 665] Location in patent:supporting information

100-83-4
648 suppliers
$5.00/10g
2973-80-0
396 suppliers
$6.00/1g

196081-71-7
145 suppliers
$9.00/1g