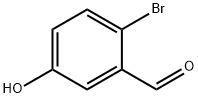
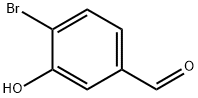
2-BROMO-5-HYDROXYBENZALDEHYDE synthesis
- Product Name:2-BROMO-5-HYDROXYBENZALDEHYDE
- CAS Number:2973-80-0
- Molecular formula:C7H5BrO2
- Molecular Weight:201.02

100-83-4

2973-80-0
General procedure for the synthesis of 2-bromo-5-hydroxybenzaldehyde from m-hydroxybenzaldehyde: 3-hydroxybenzaldehyde (120 g, 0.98 mol) was suspended in 2400 mL of dichloromethane (CH2Cl2) in a 5 L four-necked, round-bottomed flask fitted with an overhead stirrer, a temperature probe, a dosing funnel, and a condenser tube. The mixture was heated to 35-40 °C to completely dissolve the raw material. Bromine (52 mL, 1.0 mol, 1.02 eq.) was added slowly dropwise through the addition funnel, controlling the rate of dropwise acceleration to maintain the reaction temperature between 35-38°C. After dropwise addition, the reaction mixture was stirred at 35 °C overnight. Subsequently, the mixture was slowly cooled to -5-0 °C over a period of 2 h and stirring was continued for 1 h at this temperature. The precipitated solid was collected by filtration through a Brinell funnel and the filter cake was washed with 400 mL of cold 1:1 n-heptane/dichloromethane mixture. The resulting gray solid was dried under vacuum at room temperature (0.2 mmHg). The yield was 124.3 g in 63% yield.
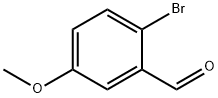
7507-86-0
192 suppliers
$7.00/1g

2973-80-0
399 suppliers
$6.00/1g
Yield:2973-80-0 90.9%
Reaction Conditions:
with boron tribromide in dichloromethane at 0 - 25; for 3 h;
Steps:
343B.1
To a mixture of 2-bromo-5-methoxybenzaldehyde (2000.0 mg, 9.3 mmol) indichioromethane (10 mL) was added boron tribromide (2M in DCM; 4.65 mL, 9.3 mmol) slowly at 0 °C. The reaction was warmed up to 25 °C and stirred for 3 h. The reaction was quenched with water (10 mL) at 0°C and extracted with EtOAc (50 mL). The organic layer was washed with water (50 mL x 2) and brine (50 mL), dried over Mg504 and concentrated. The residue was purified by flash column chromatography (petroleum ether) to afford 2-bromo-5-hydroxybenzaldehyde (1.7 g, 90.9% yield) as a colorless oil.
References:
WO2017/84630,2017,A1 Location in patent:Paragraph 00976

100-83-4
649 suppliers
$5.00/10g

2973-80-0
399 suppliers
$6.00/1g
14472-14-1
268 suppliers
$10.00/5g

2973-80-0
399 suppliers
$6.00/1g

100-83-4
649 suppliers
$5.00/10g

2973-80-0
399 suppliers
$6.00/1g
3111-51-1
63 suppliers
$55.00/25mg
20035-32-9
96 suppliers
$57.00/250mg

100-83-4
649 suppliers
$5.00/10g

2973-80-0
399 suppliers
$6.00/1g

20035-32-9
96 suppliers
$57.00/250mg