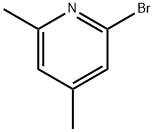
2-BROMO-4,6-DIMETHYLPYRIDINE synthesis
- Product Name:2-BROMO-4,6-DIMETHYLPYRIDINE
- CAS Number:4926-26-5
- Molecular formula:C7H8BrN
- Molecular Weight:186.05
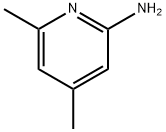
5407-87-4

4926-26-5
General procedure for the synthesis of 2-bromo-4,6-dimethylpyridine from 2-amino-4,6-dimethylpyridine: A 48% aqueous hydrobromic acid solution (Aldrich, 65 mL, 1.2 mol, 10 eq.) was cooled to -5 °C and 4,6-dimethylpyridin-2-amine (Aldrich, 15.0 g, 0.12 mol, 1.0 eq.) was added. Bromine (Aldrich, 19.7 mL, 0.38 mol, 3.1 eq.) was added slowly and dropwise with mechanical stirring to the thick white salt mixture formed. The resulting red mixture was treated with aqueous sodium nitrite (32 mL water, Aldrich, 22.1 g, 0.32 mol, 2.6 eq.) for 1 hr below 5 °C. The temperature was maintained below 5°C during the nitrite addition process, followed by a gradual warming to 20°C over 2 hr. The reaction mixture was adjusted to pH 14 with aqueous sodium hydroxide and extracted with methyl tert-butyl ether (MTBE). The organic phase was washed sequentially with water and brine, dried over magnesium sulfate, filtered and concentrated under reduced pressure. The crude product (29 g of red oily material) was purified by fast chromatography (silica gel, 350 g) using 2-7% ethyl acetate-cyclohexane as eluent to give an orange oily product (11.0 g, 48% yield). The product was purified by 1H NMR (DMSO-d6, 300 MHz) δ 7.30 (1H, s), 7.13 (1H, s), 2.39 (3H, s), 2.26 (3H, s); 13C NMR (DMSO-d6, 75 MHz) δ 159.4, 151.3, 140.9, 125.7, 123.4, 23.7, 20.3 ; ESI-MS m/z 186/188 ([M+H]+).

5407-87-4
208 suppliers
$9.00/1g

4926-26-5
123 suppliers
$18.00/250mg
Yield:4926-26-5 48%
Reaction Conditions:
Stage #1:2-Amino-4,6-dimethylpyridine with hydrogen bromide;bromine in water at -5;Acidic aqueous solution;
Stage #2: with sodium nitrite in water at 5; for 1 h;Acidic aqueous solution;
Stage #3: in water at 0 - 20; for 2 h;Acidic aqueous solution;
Steps:
4.a Example 4 (a): 2-Bromo-4, 6-dimethyl-pyridine
A solution of 48% HBr (aq) (ALDRICH, 65 mL, 1.2 mol, 10 eq) was cooled to-5°C and treated with 4, 6-DIMETHYL-PYRIDIN-2-YLAMINE (Aldrich, 15.0 g, 0.12 mol. 1.0 eq). The thick white salt mixture was stirred with a mechanical stirrer while bromine (ALDRICH, 19.7 mL, 0.38 mol, 3.1 eq) was added dropwise. The resultant red mixture was treated with an aqueous solution (32 mL H2O) of NAN02 (ALDRICH, 22.1 G, 0.32 mol, 2.6 eq) over one hour. The temperature was maintained below 5°C during the nitrite addition, and then gradually warmed to 20°C over 2 hours. The reaction mixture was adjusted to pH 14 with NAOH (aq), and extracted with MTBE. The organic extracts were washed with water, brine, dried over magnesium sulfate, filtered and concentrated under reduced pressure. The crude product (29 G of a red oil) was purified by flash chromatography (silica, 350 g) and eluted with 2-7% ethyl acetate-cyclohexane, which gave an orange oil (11.0 g, 48%). 1H NMR (DMSO-D6, 300 MHz) 5 7.30 (1H, s), 7.13 (1H, s), 2.39 (3H, s), 2.26 (3H, S).'3C NMR (DMSO-D6, 75 MHz) 5 159.4, 151.3, 140.9, 125.7, 124. 0, 23.7, 20.3. ESI m/z 186/188 (M + H)+.
References:
PFIZER INC. WO2004/56806, 2004, A1 Location in patent:Page 50