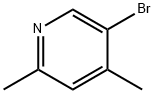
5-BROMO-2,4-DIMETHYLPYRIDINE synthesis
- Product Name:5-BROMO-2,4-DIMETHYLPYRIDINE
- CAS Number:27063-92-9
- Molecular formula:C7H8BrN
- Molecular Weight:186.05
3430-26-0

75-16-1

27063-92-9
A solution of 2,5-dibromo-4-methylpyridine (2.51 g, 10.0 mmol) in anhydrous THF (50 mL) was added to a three-necked flask, followed by tetrakis(triphenylphosphine)palladium (1.15 g, 1.0 mmol). The reaction mixture was cooled to 0 °C under nitrogen protection and stirred. A THF solution of methylmagnesium bromide (15 mL, 15.0 mmol, 1 mol/L) was added slowly and dropwise. After the dropwise addition, the reaction mixture was heated to reflux for 3 hours. After completion of the reaction, it was cooled to room temperature, the reaction was quenched with 1N hydrochloric acid and extracted with ethyl acetate (100 mL x 3). The organic layers were combined, washed sequentially with water (50 mL x 2) and saturated saline (50 mL) and dried over anhydrous sodium sulfate. After concentration under reduced pressure, the crude product was purified by Biotage fast chromatography (petroleum ether/ethyl acetate = 5%-10%) to afford 2,4-dimethyl-5-bromopyridine (301 mg, 16.2%) as a colorless oil.1H NMR (400 MHz, CDCl3) δppm: 2.35 (d, 3H), 2.47 (s, 3H), 7.04 ( s, 1H), 8.50 (s, 1H).

3430-26-0
338 suppliers
$10.00/1g

75-16-1
275 suppliers
$12.00/10ml

27063-92-9
90 suppliers
$65.00/50 mg
Yield: 16.2%
Reaction Conditions:
Stage #1:2,5-Dibromo-4-methylpyridine with tetrakis(triphenylphosphine) palladium(0) in tetrahydrofuran at 0;Inert atmosphere;
Stage #2:methylmagnesium bromide in tetrahydrofuran for 3 h;Reflux;
Steps:
5-Bromo-2,4-dimethylpyridine (B12.2)
To a three-neck flask, was placed B12.1 (2.51 g, 10.0 mmol) in dry THF (50 mL), Pd(PPh3)4 (1.15 g, 1.0 mmol). The mixture was protected under N2 and stirred at 0 oC. MeMgBr/THF (15 mL, 15.0 mmol, 1 mol/L) was added slowly. After addition, the mixture was refluxed for 3 h, cooled to rt, quenched the reaction mixture with 1N HCl and extracted with EtOAc (100 mL × 3). The organic layer was washed with H2O (50 mL × 2) and brine (50 mL), then dried over anhydrous Na2SO4 and concentrated under vacuum and the crude product was purified by Biotage flash chromatography (PE/EtOAc = 5%-10%) to afford the title compound (301 mg, 16.2%) as a colorless oil.1H NMR (400 MHz, CDCl3) δ ppm 2.35 (d, 3H), 2.47 (s, 3H), 7.04 (s, 1H), 8.50 (s, 1H).
References:
NOVARTIS AG;CHAN, Ho Man;FU, Xingnian;GU, Xiang-Ju Justin;HUANG, Ying;LI, Ling;MI, Yuan;QI, Wei;SENDZIK, Martin;SUN, Yongfeng;WANG, Long;YU, Zhengtian;ZHANG, Hailong;ZHANG, Ji Yue;ZHANG, Man;ZHANG, Qiong;ZHAO, Kehao WO2017/221092, 2017, A1 Location in patent:Paragraph 00190
1193-71-1
91 suppliers
$17.00/250mg

27063-92-9
90 suppliers
$65.00/50 mg
108-47-4
229 suppliers
$9.00/1g

27063-92-9
90 suppliers
$65.00/50 mg
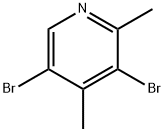
29976-20-3
40 suppliers
$54.00/100mg
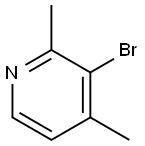
27063-93-0
102 suppliers
$37.00/100mg

108-47-4
229 suppliers
$9.00/1g

27063-92-9
90 suppliers
$65.00/50 mg

27063-93-0
102 suppliers
$37.00/100mg

29976-20-3
40 suppliers
$54.00/100mg

27063-92-9
90 suppliers
$65.00/50 mg

27063-93-0
102 suppliers
$37.00/100mg