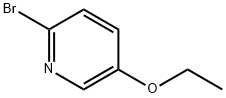
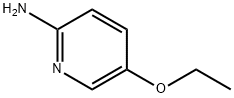
2-BROMO-5-ETHOXYPYRIDINE synthesis
- Product Name:2-BROMO-5-ETHOXYPYRIDINE
- CAS Number:42834-01-5
- Molecular formula:C7H8BrNO
- Molecular Weight:202.05
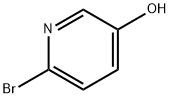
55717-45-8
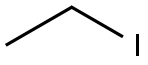
75-03-6

42834-01-5
Sodium hydride (60% dispersed in mineral oil, 0.644 g, 16.09 mmol) was added batchwise to a solution of N,N-dimethylformamide (DMF, 20 mL) of 6-bromopyridin-3-ol (2.0 g, 11.49 mmol) at room temperature and the reaction mixture was stirred for 30 min at 0 °C. Subsequently, iodoethane (1.858 mL, 22.99 mmol) was slowly added dropwise and the reaction mixture was continued to be stirred at room temperature for 3.5 hours. Upon completion of the reaction, the reaction was quenched by adding water to the mixture and the organic phase was extracted with ethyl acetate. The combined organic layers were washed sequentially with water and saturated saline, dried over anhydrous magnesium sulfate and concentrated under reduced pressure to remove the solvent. The crude product was purified by silica gel column chromatography (eluent: hexane-ethyl acetate gradient elution) to afford 2-bromo-5-ethoxypyridine (2.32 g, 100% yield). The product was characterized by 1H-NMR (CDCl3): δ 8.03 (1H, d, J = 2.75 Hz), 7.35 (1H, d, J = 8.69 Hz), 7.07 (1H, dd, J = 8.62, 2.97 Hz), 4.05 (2H, q, J = 6.91 Hz), 1.43 (3H, t, J = 6.94 Hz).

55717-45-8
288 suppliers
$5.00/250mg

75-03-6
369 suppliers
$11.00/5g

42834-01-5
46 suppliers
$80.00/1g
Yield:42834-01-5 100%
Reaction Conditions:
Stage #1: 6-bromo-pyridin-3-olwith sodium hydride in N,N-dimethyl-formamide at 20; for 0.5 h;
Stage #2: ethyl iodide in N,N-dimethyl-formamide at 20; for 3.5 h;
Steps:
12.1
Reference Example 12 [0441] Step 1 [0442] To a solution of Compound vii-2 (2.0g, 11.49mmol) in DMF (20mL) was added sodium hydride (0.644g, 16.09mmol) at room temperature, and the mixture was stirred for 30 minutes. Iodoethane (1.858mL, 22.99mmol) was added to the mixture and the resulting mixture was stirred for additional 3.5 hours at room temperature. To the reaction mixture was added water, and the mixture was extracted with ethyl acetate. The organic layer was washed by water and brine, dried over anhydrous magnesium sulphate, and concentrated in vacuo. The resulting residue was purified by silica gel column chromatography (hexane-ethyl acetate) to give Compound vii-3 (2.32g, Yield 100%). [0443] 1H-NMR (CDCl3) δ: 8.03 (1H, d, J = 2.75 Hz), 7.35 (1H, d, J = 8.69 Hz), 7.07 (1H, dd, J = 8.62, 2.97 Hz), 4.05 (2H, q, J = 6.91 Hz), 1.43 (3H, t, J = 6.94 Hz).
References:
EP2752410,2014,A1 Location in patent:Paragraph 0441-0443

55717-45-8
288 suppliers
$5.00/250mg
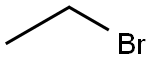
74-96-4
442 suppliers
$9.00/5g

42834-01-5
46 suppliers
$80.00/1g
89943-11-3
55 suppliers
$65.00/100mg

42834-01-5
46 suppliers
$80.00/1g