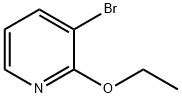
3-BROMO-2-ETHOXYPYRIDINE synthesis
- Product Name:3-BROMO-2-ETHOXYPYRIDINE
- CAS Number:57883-25-7
- Molecular formula:C7H8BrNO
- Molecular Weight:202.05
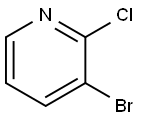
52200-48-3

64-17-5
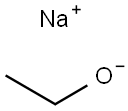
141-52-6

57883-25-7
3-Bromo-2-chloropyridine (10.0 g) was used as a raw material, which was dissolved in anhydrous ethanol (100 mL), followed by the slow addition of an ethanol solution of 21% sodium ethoxide (70.8 mL). The reaction mixture was heated to reflux and kept reacting overnight. After completion of the reaction, the solvent was removed by rotary evaporator under reduced pressure. The residue was dissolved in ethyl acetate and washed sequentially with saturated aqueous sodium bicarbonate and aqueous ammonium chloride. The organic layer was dried with anhydrous sodium sulfate, filtered and the solvent evaporated to give the crude product. The crude product was purified by silica gel column chromatography with the eluent of cyclohexane/ethyl acetate (10:1, v/v) to give 3-bromo-2-ethoxypyridine (7.26 g, 69% yield) as a yellow oil. The product was characterized by 1H-NMR (400 MHz, DMSO-d6): δ[ppm] = 1.34 (t, 3H), 4.37 (q, 2H), 6.93 (dd, 1H), 8.02 (dd, 1H), 8.15 (dd, 1H).
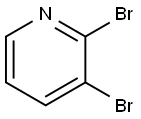
13534-89-9
328 suppliers
$6.00/1g

64-17-5
825 suppliers
$10.00/50g

57883-25-7
106 suppliers
$72.50/250mg
Yield:57883-25-7 85%
Reaction Conditions:
with sodium for 12 h;Reflux;
Steps:
3-Bromo-2-ethoxypyridine (4a); Typical Procedure
General procedure: Na (2.40 g, 105 mmol) was slowly dissolved in anhyd EtOH (100 mL)while stirring. Then, 2,3-dibromopyridine (7.11 g, 30.0 mmol) wasadded and the reaction mixture was refluxed for 12 h. After quenchingwith sat. aq NH4Cl, the product was extracted with EtOAc. Thecombined organic extracts were dried (Na2SO4) and evaporated invacuo. The crude product obtained was purified by flash chromatography(n-pentane:EtOAc 9:1) to give 4a as a yellow liquid; yield: 5.15g (25.5 mmol, 85%).IR (Diamond-ATR, neat): 2978, 1581, 1471, 1444, 1429, 1381, 13511314, 1297, 1251, 1243, 1068, 1035, 1019, 928, 781, 750, 690 cm-1.1H NMR (400 MHz, CDCl3): = 8.07 (dd, J = 4.9, 1.7 Hz, 1 H), 7.79 (dd,J = 7.6, 1.7 Hz, 1 H), 6.74 (dd, J = 7.6, 4.9 Hz, 1 H), 4.43 (q, J = 7.0 Hz, 2H), 1.43 (t, J = 7.1 Hz, 3 H).13C NMR (101?MHz, CDCl 3): = 159.9, 145.6, 141.7, 117.6, 107.3, 63.0,14.7.MS (EI, 70 eV): m/z (%) = 188 (98), 186 (100), 175 (34), 153 (35), 159(19), 157 (19), 147 (57), 145 (59), 78 (37).HRMS (EI): m/z [M - CH3]+ calcd for C6H5BrNO: 185.9555; found:185.9548
References:
Heinz, Benjamin;Djukanovic, Dimitrije;Siemens, Fiona;Idriess, Mohamed;Martin, Benjamin;Knochel, Paul [Synthesis,2021,vol. 54,# 22,p. 5055 - 5063]
36178-05-9
236 suppliers
$15.00/5g

64-17-5
825 suppliers
$10.00/50g

57883-25-7
106 suppliers
$72.50/250mg

52200-48-3
371 suppliers
$11.00/1g

64-17-5
825 suppliers
$10.00/50g

57883-25-7
106 suppliers
$72.50/250mg

52200-48-3
371 suppliers
$11.00/1g

57883-25-7
106 suppliers
$72.50/250mg

52200-48-3
371 suppliers
$11.00/1g

141-52-6
506 suppliers
$10.00/5g

57883-25-7
106 suppliers
$72.50/250mg