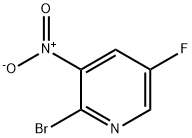
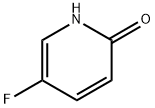
2-Bromo-5-fluoro-3-nitropyridine synthesis
- Product Name:2-Bromo-5-fluoro-3-nitropyridine
- CAS Number:652160-72-0
- Molecular formula:C5H2BrFN2O2
- Molecular Weight:220.98
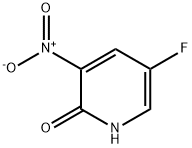
136888-20-5

652160-72-0
General procedure for the synthesis of 2-bromo-5-fluoro-3-nitropyridine from 2-hydroxy-3-nitro-5-fluoropyridine: 117 g of 2-hydroxy-3-nitro-5-fluoropyridine was divided into 4 batches (30 g x 3 and 27 g x 1) and treated with phosphorus tribromide (3 equiv; 163 g x 3 and 155 g x 1) and catalytic amount of N,N-dimethylformamide (15 mL), respectively. DMF was carefully added at room temperature with attention to gas release.After 5 min, the reaction mixture was heated to 110 °C and maintained for 3 h. The reaction mixture was then purified by LC/MS. Complete consumption of the feedstock was confirmed by LC/MS monitoring. After cooling the reaction mixture to room temperature, the reaction flask was placed in an ice bath. Ice was added to the flask slowly and carefully in batches, noting the escape of gas due to HBr generation. The resulting liquid and black solid were poured into the beaker containing ice. Ethyl acetate was added and the mixture was subsequently extracted several times with ethyl acetate. The organic layer was washed sequentially with saturated sodium bicarbonate solution, water and brine, dried over anhydrous sodium sulfate and filtered. The product was dried overnight in a vacuum pump to give 123 g of 2-bromo-5-fluoro-3-nitropyridine as a brown solid in 77% yield. Note: The reaction was completed in 1 h. 1H NMR (δ, CDCl3): 8.52 (m, 1H), 7.93 (m, 1H).

136888-20-5
142 suppliers
$8.00/100mg

652160-72-0
102 suppliers
$15.00/250mg
Yield: 77%
Reaction Conditions:
with N,N-dimethyl-formamide;phosphorus(V) oxybromide at 20 - 110; for 3.08333 h;
Steps:
A total of 117 g of 5-80 was divided in 4 batches of 30 g×3 and 27 g×1 and treated with POBr3 (3 equiv.; 163 g×3 and 155 g×1) and a catalytic amount of DMF (15 ml) at rt (DMF was added carefully gas evolution). After 5 min. at room temperature, the solutions were heated at 110° C. for 3 hr. LC/MS showed starting material had been consumed. The reaction mixtures were allowed to cool to rt. The reaction flasks were placed in an ice bath; and then ice was added very slowly and carefully portionwise into the flask, gas evolution was due to HBr formation; the liquid and black solid that formed was poured into a beaker with ice. EtOAc was added and the mixture was then extracted several times with EtOAc. The organic layer was washed with saturated aq. NaHCO3; H2O and brine; dried over Na2SO4 and filtered. The product was dried in the pump overnight to provide 123 g of 6-80 as a brown solid (77% yield). [1498] Note: Reaction is completed within 1 h. [1499] 1HNMR(δ, CDCl3):8.52 (m, 1H), 7.93 (m, 1H).
References:
Wang, Tao;Zhang, Zhongxing;Meanwell, Nicholas A.;Kadow, John F.;Yin, Zhiwei;Xue, Qiufen May;Regueiro-Ren, Alicia;Matiskella, John D.;Ueda, Yasutsugu US2004/110785, 2004, A1 Location in patent:Page 212
6628-77-9
357 suppliers
$8.00/5g

652160-72-0
102 suppliers
$15.00/250mg
51173-04-7
236 suppliers
$8.00/1g

652160-72-0
102 suppliers
$15.00/250mg
51173-05-8
215 suppliers
$6.00/250mg

652160-72-0
102 suppliers
$15.00/250mg