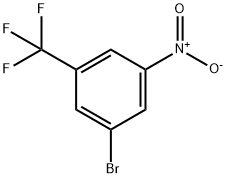
3-BROMO-5-NITROBENZOTRIFLUORIDE synthesis
- Product Name:3-BROMO-5-NITROBENZOTRIFLUORIDE
- CAS Number:630125-49-4
- Molecular formula:C7H3BrF3NO2
- Molecular Weight:270
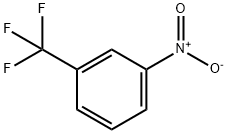
98-46-4

630125-49-4
Example 14 Synthesis of 1-bromo-3-nitro-5-trifluoromethylbenzene (XI): To a solution of 1-nitro-3-trifluoromethylbenzene (41.1 mL, 300 mmol, 97%, purchased from Aldrich) in dichloromethane (240 mL) was slowly added 98% sulfuric acid (45.7 mL, 840 mmol) over a 10 minute period. The two-phase mixture obtained with vigorous stirring was heated to 35 °C and 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione (total 53.1 g, 180 mmol) was added in six equal portions over 5 hours. The mixture was continued to be stirred at 35 °C for 19 h. HPLC analysis showed more than 97% conversion of the starting material. After the reaction mixture was cooled to room temperature, it was added dropwise to a stirred aqueous 2 M NaOH solution (210 mL) at 0-5 °C over 20 min, during which time it was cooled in an ice-water bath, and the internal temperature was briefly raised to about 35 °C. The reaction mixture was then cooled to room temperature, and the internal temperature was raised to about 35 °C. The reaction mixture was then cooled to room temperature. The two layers were separated and the aqueous layer was extracted with hexane (3 x 200 mL). The combined organic layers were washed sequentially with water (200 mL), 5% aqueous sodium bisulfite (2 x 200 mL), 8% aqueous NaHCO3 (200 mL), and 10% aqueous NaCl (200 mL), followed by evaporation of the solvent at 45 °C under reduced pressure. The resulting liquid was distilled at 0.71 mbar and a bath temperature of 70-80 °C to give 1-bromo-3-nitro-5-trifluoromethylbenzene as a light yellow liquid in 89.6% yield (1H-NMR purity about 95%).1H-NMR (400 MHz, CDCl3): δ 8.11 (m, 1H), 8.45 (m, 1H), 8.58-8.59 (m, 1H). 1H). Boiling point: ca. 68 °C (0.71 mbar).

98-46-4
258 suppliers
$19.00/5g

630125-49-4
257 suppliers
$14.00/1g
Yield:630125-49-4 89.6%
Reaction Conditions:
Stage #1: 3-trifluoromethylnitrobenzenewith sulfuric acid in dichloromethane;water at 35; for 0.166667 h;
Stage #2: with 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione in dichloromethane;water at 35; for 24 h;
Stage #3: with sodium hydroxide in dichloromethane;water at 0 - 35; for 0.333333 h;
Steps:
14
Example 14 1-Bromo-3-nitro-5-trifluoromethyl-benzene (Xl)To a solution of 1-nitro-3-trifluoromethyl-benzene (41.1 mL, 300 mmol, 97%, purchased from Aldrich) in dichloromethane (240 mL) is added 98% sulfuric acid (45.7 mL, 840 mmol) over 10 minutes. The vigorously stirred resulting biphasic mixture is warmed to 35°C and 1 ,3-dibromo-5,5-dimethyl-imidazolidine-2,4-dione (53.1 g in total, 180 mmol) is added in six equal portions over five hours. The mixture is stirred at 35°C for additional19 hours. Thereafter, more than 97% of the starting material is converted according to HPLC analysis. The reaction mixture is allowed to cool to room temperature and added over20 minutes to a stirred 2 M aqueous NaOH solution (210 mL) of 0-5°C while cooling with an ice-water bath. The internal temperature rises temporarily to about 35°C. The two layers are separated. The aqueous layer is extracted with hexane (3 x 200 mL). The combined organic layers are washed with water (200 mL), 5% aqueous sodium metabisulfite solution (2 x EPO
References:
WO2006/135640,2006,A2 Location in patent:Page/Page column 25-26
320-94-5
235 suppliers
$9.00/1g

630125-49-4
257 suppliers
$14.00/1g

479411-92-2
20 suppliers
$59.29/250mg

630125-49-4
257 suppliers
$14.00/1g
401-78-5
396 suppliers
$5.00/5g

630125-49-4
257 suppliers
$14.00/1g
98-08-8
298 suppliers
$9.00/1g

630125-49-4
257 suppliers
$14.00/1g