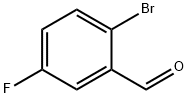
2-Bromo-5-fluorobenzaldehyde synthesis
- Product Name:2-Bromo-5-fluorobenzaldehyde
- CAS Number:94569-84-3
- Molecular formula:C7H4BrFO
- Molecular Weight:203.01
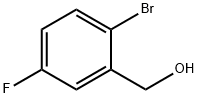
202865-66-5

94569-84-3
General procedure for the synthesis of 2-bromo-5-fluorobenzaldehyde from 2-bromo-5-fluorobenzyl alcohol: to a solution of 2-bromo-5-fluorobenzyl alcohol (0.852 g, 4.156 mmol) in dichloromethane (15 mL) was added manganese dioxide (4.254 g, 85%, 41.56 mmol). The reaction mixture was stirred at room temperature for 48 h. The solid was subsequently separated by filtration and washed with dichloromethane. The filtrate was concentrated to give 777 mg of 2-bromo-5-fluorobenzaldehyde (92% yield). Subsequently, the newly prepared 2-bromo-5-fluorobenzaldehyde (0.777 g, 3.828 mmol) was dissolved in anhydrous tetrahydrofuran (10 mL) and the solution was cooled to 0 °C. At low temperature, trifluoromethyltrimethylsilane (1.13 mL, 7.656 mmol) and tetrabutylammonium fluoride (0.020 g, 0.076 mmol) were added sequentially. After that, the reaction mixture was slowly warmed up to room temperature and stirring was continued at that temperature for 5 hours. After completion of the reaction, the reaction mixture was diluted with ethyl acetate, washed sequentially with water and brine and dried with magnesium sulfate. The solvent was removed under reduced pressure to afford 1.1 g (90% purity) of the crude product of 2-bromo-5-fluorophenyl-2,2,2-trifluoroethanol, which could be used in subsequent steps without further purification.

202865-66-5
246 suppliers
$6.00/1g

94569-84-3
312 suppliers
$16.00/5g
Yield:94569-84-3 92%
Reaction Conditions:
with manganese(IV) oxide in dichloromethane at 20; for 48 h;
Steps:
6.24
To the solution of (2-bromo-5-fluoro-phenyl)methanol (0.852 g, 4.156 mmol) in DCM (15 ml) was added MnO2 (4.254 g, 85%, 41.56 mmol). The mixture was stirred at room temperature for two days, and then filtered and washed with DCM. The filtrate was concentrated to afford 777 mg 2-bromo-5-fluoro-benzaldehyde (92% yield). The newly made aldehyde (0.777 g, 3.828 mmol) was then dissolved in anhydrous THF (10 ml) and cooled to 0° C. Trifluoromethyl trimethylsilane (1.13 ml, 7.656 mmol) was added, and followed by tetrabutyl ammonium fluoride (0.020 g, 0.076 mmol). The temperature was then allowed to warm to room temperature. The mixture was stirred for 5 h at room temperature, then diluted with ethyl acetate, washed with water, brine and dried by MgSO4. The solvent was removed under reduced pressure to give 2-bromo-5-fluoro-phenyl)2,2,2-trifluoro-ethanol, 1.1 g (90% purity) as a crude product, which was used for the next step without further purification.
References:
Jin, Haihong;Shi, Zhi-Cai;Tunoori, Ashok;Wang, Ying;Zhang, Chengmin;Devasagayaraj, Arokiasamy US2008/153852, 2008, A1 Location in patent:Page/Page column 23
452-63-1
342 suppliers
$11.00/25g

94569-84-3
312 suppliers
$16.00/5g
112399-50-5
237 suppliers
$7.00/5g

94569-84-3
312 suppliers
$16.00/5g

112399-50-5
237 suppliers
$7.00/5g

94569-84-3
312 suppliers
$16.00/5g

202865-66-5
246 suppliers
$6.00/1g

202865-66-5
246 suppliers
$6.00/1g
394-28-5
307 suppliers
$10.00/1g

94569-84-3
312 suppliers
$16.00/5g