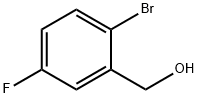
2-Bromo-5-fluorobenzyl alcohol synthesis
- Product Name:2-Bromo-5-fluorobenzyl alcohol
- CAS Number:202865-66-5
- Molecular formula:C7H6BrFO
- Molecular Weight:205.02
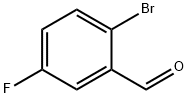
94569-84-3

202865-66-5
General procedure for the synthesis of 2-bromo-5-fluorobenzyl alcohol from 2-bromo-5-fluorobenzaldehyde: To a solution of 2-bromo-5-fluorobenzaldehyde (75.0 g, 369 mmol) in ethanol (375 mL) was slowly added a solution of sodium borohydride (4.47 g, 118 mmol) in water (9 mL) at 0 °C. The reaction mixture was kept stirred at 0°C for 1 hour. Upon completion of the reaction, the mixture was treated with water and ethyl acetate and extracted with ethyl acetate. The combined organic extracts were washed with saturated brine and dried over anhydrous sodium sulfate. The solvent was removed by concentration under reduced pressure to give 75.6 g of 2-bromo-5-fluorobenzyl alcohol (yield = 99.8%) as a colorless solid.1H NMR (CDCl3) δ 7.50-7.46 (1H, m), 7.29-7.25 (1H, m), 6.92-6.85 (1H, m), 4.72 (2H, s).

94569-84-3
312 suppliers
$16.00/5g

202865-66-5
246 suppliers
$6.00/1g
Yield:202865-66-5 99.8%
Reaction Conditions:
with sodium tetrahydroborate in ethanol;water at 0 - 20; for 1 h;
Steps:
To a solution of compound A2-1 (75.0 g, 369 mmol) in EtOH (375 rnL) was added a solution OfNaBH4 (4.47 g, 118 mmol) in water (9 mL) at 0°C, and the mixture was stirred at rt for 1 h. The mixture was treated with water and EtOAc, and extracted with EtOAc. The extract was washed with brine, dried over Na2SO4. The solvent was concentrated in vacuo to give 75.6 g of compound A2-2 (yield = 99.8 %) as a colorless solid. 1H NMR (CDCl3) δ 7.5O-7.46(1H, m), 7.29-7.25(1H, m), 6.92-6.85(1H, m), 4.72(2H, s).
References:
WO2007/19098,2007,A2 Location in patent:Page/Page column 44
133188-66-6
10 suppliers
inquiry

202865-66-5
246 suppliers
$6.00/1g
112399-50-5
237 suppliers
$7.00/5g

202865-66-5
246 suppliers
$6.00/1g
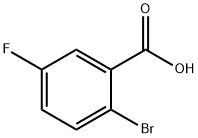
394-28-5
307 suppliers
$10.00/1g

202865-66-5
246 suppliers
$6.00/1g
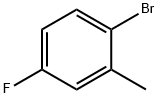
452-63-1
342 suppliers
$11.00/25g

202865-66-5
246 suppliers
$6.00/1g