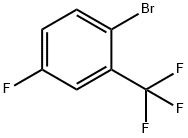
2-Bromo-5-fluorobenzotrifluoride synthesis
- Product Name:2-Bromo-5-fluorobenzotrifluoride
- CAS Number:40161-55-5
- Molecular formula:C7H3BrF4
- Molecular Weight:243
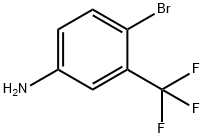
393-36-2

40161-55-5
The 1L stainless steel reactor was cooled to -10 °C and then 450 g of anhydrous hydrofluoric acid and 6.0 g of anhydrous potassium fluoride were added to the reactor. Subsequently, 248 g of 5-amino-2-bromobenzotrifluoride was added and 75.5 g of sodium nitrite was added slowly, ensuring that the temperature of the reaction system did not exceed 5 °C. Stirring was done for 30 minutes to complete the diazotization reaction. The temperature of the reaction system was raised to 60°C and maintained, at which time the fluorinated diazonium salt began to decompose slowly and release gases, which were absorbed by the base after passing through the cooling buffer. After the reaction is complete and no more gas is released, the excess hydrofluoric acid in the reactor is recovered and the mixture is cooled to 0°C. A 10% KOH solution was added to the reactor to neutral and steam distillation was carried out to obtain 233 g of crude 2-bromo-5-fluorobenzotrifluoride. The distillation was carried out by filling a distillation column and the 110°C-120°C fractions were collected at a pressure of 40 mmHg to obtain 209 g of a colorless transparent liquid, 2-bromo-5-fluorobenzotrifluoride, with a GC purity of 99% and a yield of 86.8%.
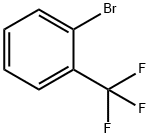
392-83-6
330 suppliers
$6.00/10g
5942-30-3
0 suppliers
inquiry
Yield:5942-30-3 96.7%
Reaction Conditions:
with potassium fluoride;potassium tetrafluorocobaltate in dimethyl sulfoxide; for 5 h;Reflux;
Steps:
1-7
Add 2-bromobenzotrifluoride (2.3g), DMSO (60mL), potassium fluoride (2.5g) and KCoF4 (7.0g) in a 100mL flask, and carry out the reaction under heating and reflux for 5 hours. After evaporating part of the solvent 100 mL of water was added, followed by extraction with dichloromethane, and column chromatography to obtain 2.4 g of 2-bromo-5-fluorobenzotrifluoride with a yield of 96.7%.
References:
CN111362775,2020,A Location in patent:Paragraph 0025; 0027-0028; 0030-0031; 0033-0034; 0036-0037
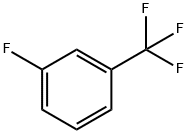
401-80-9
302 suppliers
$5.00/10g

5942-30-3
0 suppliers
inquiry

393-36-2
313 suppliers
$6.00/1g

5942-30-3
0 suppliers
inquiry
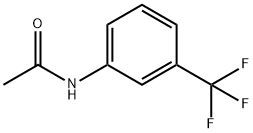
351-36-0
120 suppliers
$29.00/25g

5942-30-3
0 suppliers
inquiry
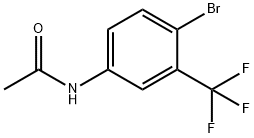
41513-05-7
85 suppliers
$11.00/250mg

5942-30-3
0 suppliers
inquiry