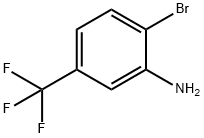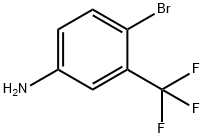
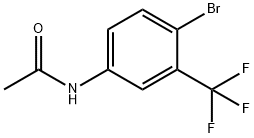
4-Bromo-3-(trifluoromethyl)aniline synthesis
- Product Name:4-Bromo-3-(trifluoromethyl)aniline
- CAS Number:393-36-2
- Molecular formula:C7H5BrF3N
- Molecular Weight:240.02
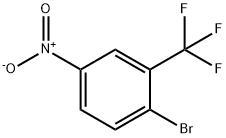
367-67-9

393-36-2
General procedure for the synthesis of 5-amino-2-bromobenzotrifluoride from 2-bromo-5-nitrobenzotrifluoride: 300 mL of methanol, 284 g of 2-bromo-5-nitrobenzotrifluoride, 20 g of Raney Ni Catalyst, and 1.0 g of piperazine were added to a 1 L high pressure hydrogenation reactor. The air in the reactor was replaced with hydrogen 3-4 times, followed by the hydrogenation reaction at a hydrogen pressure of 0.8-1.0 MPa and 80-90 °C until no more hydrogen was consumed. Upon completion of the reaction, the reaction mixture was cooled and filtered to remove the Raney Ni catalyst. Methanol and water were removed by distillation under reduced pressure, and the final product was obtained by simple distillation as a pale yellow liquid product, 5-amino-2-bromobenzotrifluoride, 250 g, with a GC purity of 95% and a yield of 98.8%.The Raney Ni catalyst and the solvent, methanol, can be recovered for reuse.
98-16-8
395 suppliers
$9.00/5g

393-36-2
313 suppliers
$6.00/1g
Yield:393-36-2 92%
Reaction Conditions:
with N-Bromosuccinimide in N,N-dimethyl-formamide at 20; for 3 h;
Steps:
General procedure for the synthesis of 4-Bromo-3-(substituted) aniline (3a-b)
General procedure: To a solution of substituted aniline 2a or 2b in DMF 100 ml was added to a solution of NBS (92 mmol, 1.0 eq) in DMF 100 ml dropwise. The reaction mixture was stirred at room temperature for 3 h, then diluted with ethyl acetate 500 ml and washed with brine 2 x 150 ml. The organic phase was dried Na2SO4, filtered and concentrated to give the title compound 3a or 3b as brownish solid, 90-92 % yield. To a solution of 2a or 2b (0.8 mmol) in the selected solvent (1 ml), a solution of NBS (0.8 mmol) in the same solvent (1 ml) was added batch wise at room temperature, and the reaction was monitored by LC-MS/MS. Mixtures were obtained with traces of dibrominated products. 4-Bromo-3-(trifluoromethyl) aniline (3a) Yield: 92 %; mp 48-50 °C; brownish powder; IR (KBr) ν max: 3485-3362 (N-Hstretch), 2219 (C:N), 1627 (N-Hbend), 1615 1566, 1509, 1467 (C=Carom), 1361, 1185, 1121, 1044 (C-Hbend), 1276, 550 (CF3) cm-1; 1H NMR (CDCl3, 400 MHz, ppm): δ 7.38 (d, 1H, J = 8.8 Hz, C5-H), 6.95 (d, 1H, J = 2.8 Hz, C2-H), 6.63 (dd, 1H, J = 2 Hz, J = 8.4 Hz, C6-H), 3.84 (br s, 2H, -NH2); 13C NMR (100 MHz, CDCl3, ppm): δ 145.7 (C1),135.5 (C5), 130.6 (C3), 124.3 (C6), 121.6 (CF3), 119.07 (C2), 114.1 (C4); HRMS (ESI): m/z calculated for C7H5 79BrF3N [M-H]-: 237.94847; Found: 237.94478; Calculated for C7H5 81BrF3N [M-H]-: 239.94620; Found: 239. 94250.
References:
Elancheran;Saravanan;Choudhury, Bhaswati;Divakar;Kabilan;Ramanathan;Das, Babulal;Devi;Kotoky, Jibon [Medicinal Chemistry Research,2016,vol. 25,# 4,p. 539 - 552]

367-67-9
328 suppliers
$10.00/5 g

393-36-2
313 suppliers
$6.00/1g
41513-05-7
85 suppliers
$11.00/250mg

393-36-2
313 suppliers
$6.00/1g
392-83-6
330 suppliers
$6.00/10g

393-36-2
313 suppliers
$6.00/1g