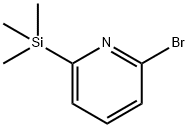
2-broMo-6-(triMethylsilyl)pyridine synthesis
- Product Name:2-broMo-6-(triMethylsilyl)pyridine
- CAS Number:59409-80-2
- Molecular formula:C8H12BrNSi
- Molecular Weight:230.18
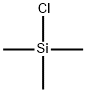
626-05-1
505 suppliers
$9.00/10g
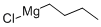
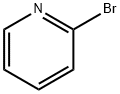
693-04-9
209 suppliers
$11.00/5g

59409-80-2
19 suppliers
$86.00/50mg
Yield:59409-80-2 38%
Reaction Conditions:
with n-butyllithium;chloro-trimethyl-silane;acetic acid in tetrahydrofuran;hexane;toluene;
Steps:
W.4 Production of (6-bromopyridin-2-yl)trimethylsilane
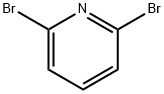
WORKING EXAMPLE 4 Production of (6-bromopyridin-2-yl)trimethylsilane n-Butylmagnesium chloride (4.00 mmol) in 2.00M tetrahydrofuran solution (2.00 mL) was added to ice-cooled n-butyllithium(8.06 mmol) in 1.55M hexane (5.20 mL). The mixture was stirred at 0° C. for 15 minutes, and 2,6-dibromo-pyridine (2.37 g, 10.0 mmol) in toluene (25 mL) was added thereto below 10° C. over a period of 10 minutes or more. The resultant suspension was stirred at 0° C. for one hour, and chlorotrimethylsilane (13 mmol, 1.65 mL)) was added thereto. After the mixture was stirred at 0° C. for one hour and at room temperature for 1.5 hours, an aqueous solution (20 mL) of 1M acetic acid was added thereto. The reaction mixture was extracted twice with ethyl acetate (50 mL). The organic phase was separated, washed with water (10 mL), dried over magnesium sulfate, and concentrated under reduced pressure to give a residue. The residue was purified by flash column chromatography on silica gel in a developing solvent system of hexane-ethyl acetate (20:1, v/v) to give the title compound (881 mg, about 38% yield) as colorless powder. 1H-NMR(CDCl3) δppm:0.32 (9H, s), 7.37 (1H, dd, J=3.2, 6.0 Hz), 7.42 (1H, dd, J=3.0, 3.2 Hz), 7.43 (1H, d, J=3.0, 3.8 Hz)
References:
US2003/130511,2003,A1