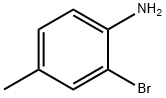
2-BROMO-N,N,4-TRIMETHYLANILINE synthesis
- Product Name:2-BROMO-N,N,4-TRIMETHYLANILINE
- CAS Number:23667-06-3
- Molecular formula:C9H12BrN
- Molecular Weight:214.1
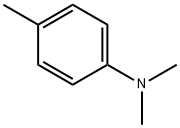
99-97-8

23667-06-3
General procedure for the synthesis of 2-bromo-N,N,4-trimethylaniline from N,N-dimethyl-p-toluidine: In a round-bottomed flask, N,N-dimethyl-p-toluidine (1 mmol) was mixed with 48% aqueous hydrobromic acid (1 mL) in dimethyl sulfoxide (DMSO, 1 mL). The reaction mixture was stirred at the indicated temperature for 1-4 hours. After completion of the reaction, it was cooled to room temperature and the pH of the reaction solution was adjusted to 7-8 with 4 M aqueous sodium hydroxide.Subsequently, the reaction mixture was extracted twice with ethyl acetate (EtOAc) and the organic phases were combined. The organic phase was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to afford the target product 2-bromo-N,N,4-trimethylaniline.

99-97-8
397 suppliers
$8.00/25g

23667-06-3
38 suppliers
$83.00/1g
Yield:23667-06-3 91%
Reaction Conditions:
with tetra-n-butylammonium hexafluoridophosphate;potassium carbonate;propargyl bromide in 1-methyl-pyrrolidin-2-one;lithium hydroxide monohydrate at 20; for 12 h;Electrolysis;regioselective reaction;
Steps:
General procedure for the bromination:
General procedure: All the electrochemical experiments were performed on IKA Electrasyn 2.0 instrument using the electrodes specified for the instrument from IKA. An electrasyn 2.0 reaction vial (10.0 mL) was charged with corresponding N,N-disubstituted aniline (1,1.0 mmol) and propargylbromide (2, 2.0 mmol), K2CO3 (1.5 mmol), a mixture of N-methyl-2-pyrrolidone and water (5:1) as solvent (10.0 mL) and Tetrabutylammonium hexafluorophosphate (0.1 M) as electrolyte. The resulting reaction mixture was subjected to the electrolysis at a constant current of 10 mA for 12h using platinum plated cathode and glassy carbon plate as anode. After the stipulated period of time the reaction mixture was removed followed by workup with water and extraction with ethylacetate. The ethylacetate layer was evaporated under vacuo and the crude material thus obtained was purified by column chromatography over silica gel using ethylacetate-hexane or DCM-hexane as the eluting solvent to isolate the pure bromination product.
References:
Chowdhury, Sushobhan;Pandey, Shubham;Gupta, Ashutosh;Kumar, Ajay [Tetrahedron,2022,vol. 120,art. no. 132902] Location in patent:supporting information

50-00-0
896 suppliers
$10.00/25g
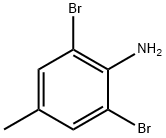
6968-24-7
215 suppliers
$10.00/5g

23667-06-3
38 suppliers
$83.00/1g
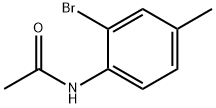
614-83-5
97 suppliers
$23.19/5g

23667-06-3
38 suppliers
$83.00/1g

87995-51-5
0 suppliers
inquiry

23667-06-3
38 suppliers
$83.00/1g