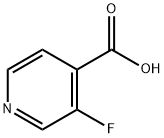
3-Fluoropyridine-4-carboxylic acid synthesis
- Product Name:3-Fluoropyridine-4-carboxylic acid
- CAS Number:393-53-3
- Molecular formula:C6H4FNO2
- Molecular Weight:141.1

124-38-9

393-53-3
3-Fluoropyridine (25 g, 257 mmol) was slowly added dropwise to a solution of tetrahydrofuran (600 mL) containing butyl lithium (270 mmol) and diisopropylamine (27.4 g, 271 mmol) at -78 °C. The reaction mixture was stirred continuously at this temperature for 1 hour. Subsequently, crushed dry ice was added to the reaction system and gradually warmed to room temperature over 1 hour. Upon completion of the reaction, the pH of the reaction solution was adjusted to 5 with an aqueous solution of hydrogen chloride and acidified. The resulting precipitate was collected by filtration and dried. The final 3-fluoroisonicotinic acid (25.2 g, 179 mmol, 70% yield) was obtained as colorless crystals.

124-38-9
134 suppliers
$175.00/23402

393-53-3
207 suppliers
$10.00/1g
Yield:393-53-3 70%
Reaction Conditions:
Stage #1:3-Fluoropyridine with n-butyllithium;diisopropylamine in tetrahydrofuran at -78; for 1 h;
Stage #2:carbon dioxide in tetrahydrofuran at -78 - 20; for 1 h;
Stage #3: with hydrogenchloride in tetrahydrofuran;water; pH=5
Steps:
1
3-Fluoropyridine (25 g, 257mmol) was added to a solution of butyllithium EPO
References:
MITSUBISHI PHARMA CORPORATION;SANOFI-AVENTIS WO2006/36015, 2006, A2 Location in patent:Page/Page column 121-122
372-47-4
383 suppliers
$5.00/1g

124-38-9
134 suppliers
$175.00/23402

393-53-3
207 suppliers
$10.00/1g

372-47-4
383 suppliers
$5.00/1g

393-53-3
207 suppliers
$10.00/1g
399-88-2
191 suppliers
$6.00/250mg

393-53-3
207 suppliers
$10.00/1g
3430-27-1
460 suppliers
$13.43/1gm:

393-53-3
207 suppliers
$10.00/1g