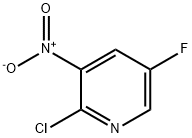
2-CHLORO-5-FLUORO-3-NITROPYRIDINE synthesis
- Product Name:2-CHLORO-5-FLUORO-3-NITROPYRIDINE
- CAS Number:136888-21-6
- Molecular formula:C5H2ClFN2O2
- Molecular Weight:176.53
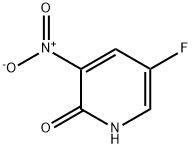
136888-20-5

136888-21-6
General procedure for the synthesis of 2-chloro-5-fluoro-3-nitropyridine from 2-hydroxy-3-nitro-5-fluoro-pyridine: 2-hydroxy-3-nitro-5-fluoro-pyridine (1.00 g, 5.33 mmol) was dissolved in acetonitrile (40 mL), followed by the addition of a solution of tetraethylammonium chloride (2.10 g, 12.65 mmol) in acetonitrile. After the mixture was clarified, phosphorus trichloride (1.94 g, 12.65 mmol) was added slowly and dropwise at room temperature. The reaction mixture was heated to 90°C and maintained for 24 hours. After completion of the reaction, the mixture was concentrated to dryness under reduced pressure. The residue was dissolved in water (100 mL) and extracted with ethyl acetate (2 x 100 mL). The organic layers were combined, dried with anhydrous sodium sulfate, filtered and concentrated to dryness under reduced pressure to give a yellow solid product (0.90 g, 69% yield), which was pure enough to be used directly in subsequent synthesis steps. Low resolution mass spectrometry (LRMS) showed m/z: 177 ([M+H]+).

136888-20-5
142 suppliers
$8.00/100mg

136888-21-6
151 suppliers
$6.00/100mg
Yield:136888-21-6 70%
Reaction Conditions:
with benzyltrimethylammonium chloride;trichlorophosphate in acetonitrile at 80; for 6 h;
Steps:
4.a
a) Synthesis of 2-chloro-5-fluoro-3-nitro-pyridine 5-fluoro-3-nitro-pyridin-2-ol (2 g, 12.7 mmol) and benzyltrimethyl ammonium chloride (1.17 g, 6.35 mmol) were dissolved in acetonitrile, and phosphorus oxychloride (3.5 ml, 38.1 mmol) was added thereto and stirred at 80° C. for 6 hours. The reaction mixture was cooled and poured into ice water to quench the reaction, and extracted with dichloromethane. The combined organic layer was washed with saturated saline solution, dried over anhydrous sodium sulfate (Na2SO4), filtered and evaporated under reduced pressure. The residue was purified by column chromatography on silica eluding with a solvent of dichloromethane:methanol=30:1. The fractions containing the product were collected and evaporated to obtain yellow liquid (1.57 g, 70%). 1H-NMR (CDCl3, 300 MHz); δ=8.56 (d, J=2.7 Hz, 1H), 8.40 (dd, J=6.5 Hz, 2.7 Hz, 1H). MS (ESI); 176.9 (M++1).
References:
US2011/28467,2011,A1 Location in patent:Page/Page column 52
884495-37-8
121 suppliers
$28.00/100mg

136888-21-6
151 suppliers
$6.00/100mg
212268-12-7
136 suppliers
$12.00/250mg

136888-21-6
151 suppliers
$6.00/100mg
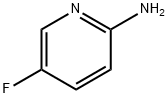
21717-96-4
433 suppliers
$10.00/1g

136888-21-6
151 suppliers
$6.00/100mg
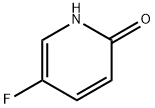
51173-05-8
215 suppliers
$6.00/250mg

136888-21-6
151 suppliers
$6.00/100mg