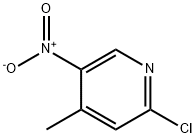
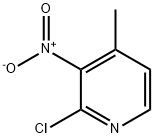
2-Chloro-4-methyl-5-nitropyridine synthesis
- Product Name:2-Chloro-4-methyl-5-nitropyridine
- CAS Number:23056-33-9
- Molecular formula:C6H5ClN2O2
- Molecular Weight:172.57
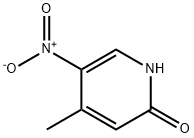
21901-41-7

23056-33-9
General procedure for the synthesis of 2-chloro-5-nitro-4-methylpyridine from 2-hydroxy-4-methyl-5-nitropyridine: 1. 2-Hydroxy-4-methyl-5-nitropyridine (500 mg, 3.24 mmol), POCl3 (0.5 mL) and PCl5 (200 mg) were mixed and stirred at 150°C for 2 hrs. 2. After completion of the reaction, cooled to room temperature, the reaction mixture was poured into ice and extracted with dichloromethane (DCM, 3 x 20 mL). 3. The organic phases were combined, washed with water to pH 7, dried over Na2SO4 and concentrated under reduced pressure. 4. The obtained residue was dried under reduced pressure to give the crude product 2-chloro-4-methyl-5-nitropyridine (480 mg, 86% yield). In the presence of PdCl2(PPh3)2 (42 mg, 0.06 mmol), P(t-Bu)3 (16 mg, 0.08 mmol) and TEA (1.5 mL), 2-chloro-4-methyl-5-nitropyridine (172 mg, 1.00 mmol) was mixed with 3-ethynyl-7,7-dimethyl-7,8-dihydroquinolin-5(6H)-one (199 mg, 1.00 mmol) in DMF (3 mL) at 100°C for 3 hours. The crude product was purified by column chromatography (silica gel) to afford the intermediate 7,7-dimethyl-3-((4-methyl-5-nitropyridin-2-yl)ethynyl)-7,8-dihydroquinolin-5(6H)-one (115 mg, 34% yield). A solution of 7,7-dimethyl-3-((4-methyl-5-nitropyridin-2-yl)ethynyl)-7,8-dihydroquinolin-5(6H)-one (114 mg, 0.34 mmol) and SnCl2-2H2O (380 mg, 1.70 mmol) in EtOH (10 mL) was stirred at reflux for 6 hours. After completion of the reaction, it was cooled to room temperature, poured into ice, adjusted to pH 7-8 with saturated aqueous NaHCO3 solution and extracted with EtOAc (3 × 30 mL). The organic phases were combined, dried over Na2SO4 and concentrated under reduced pressure. The resulting residue was purified by washing with hexane and acetonitrile to afford the target product 2-chloro-5-nitro-4-methylpyridine (60 mg, 58% yield). 1H NMR (DMSO-d6) δ: 1.07 (s, 6H), 2.08 (s, 3H), 2.60 (s, 2H), 3.02 (s, 2H), 5.51 (s, 2H), 7.23 (s, 1H), 7.90 (s, 1H), 8.17 (s, 1H), 8.80 (s, 1H). LC/MS (M+H)+ = 306.

21901-41-7
290 suppliers
$6.00/1g

23056-33-9
346 suppliers
$3.00/1g
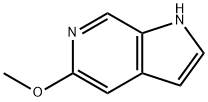
17288-53-8
102 suppliers
$14.00/100mg
Yield:17288-53-8 95%
Reaction Conditions:
with thionyl chloride in N-methyl-acetamide;dichloromethane;toluene;
Steps:
12 5-Methoxypyrrolo[2,3-c]pyridine (Compound 9)
EXAMPLE 12 5-Methoxypyrrolo[2,3-c]pyridine (Compound 9) A mixture of 4-methyl-5-nitro-1H-pyridine-2-one (5.00 g, 32.44 mmol), thionyl chloride (20 ml), and two drops of dimethylformamide was heated atreflux under nitrogen for 52 hours. The resultant orange colored solution was evaporated under reduced pressure, and a small amount of anhydrous toluene was added and then removed via evaporation under reduced pressure to remove traces of thionyl chloride. The residual oil then passed througha silica gel filter (dried at 150° C. under vacuum overnight, approximately 100 g) followed by methylene chloride (1 1). This filtrate was evaporated under reduced pressure to afford 2-chloro-4-methyl-5-nitropyridine (5.30 g, 30.71 mmol, 95%) as an orange oil, which crystallized below 0° C.; IR (CHCl3) 1605, 1550, 1520, 1450, 1360, 1345 cm-1; 1 H NMR (CDCl3) δ 9.03 (s, 1H), 7.83 (s, 1H), 2.60 (s, 3H); LRMS (m/z, relative intensity) 174 (25), 173 (19), 172 (M+, 68), 157 (74), 155 (100), 128 (27), 101 (47), 100(55], 99 (74), 90 (43), 75 (36).
References:
US5051412,1991,A

21901-41-7
290 suppliers
$6.00/1g

23056-33-9
346 suppliers
$3.00/1g
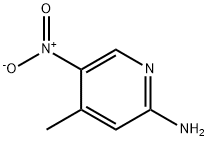
21901-40-6
300 suppliers
$6.00/1g

23056-33-9
346 suppliers
$3.00/1g
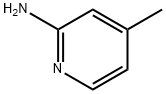
695-34-1
537 suppliers
$5.00/10g

23056-33-9
346 suppliers
$3.00/1g

695-34-1
537 suppliers
$5.00/10g

23056-33-9
346 suppliers
$3.00/1g
23056-39-5
332 suppliers
$6.00/1g