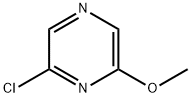
2-CHLORO-6-METHOXYPYRAZINE synthesis
- Product Name:2-CHLORO-6-METHOXYPYRAZINE
- CAS Number:33332-30-8
- Molecular formula:C5H5ClN2O
- Molecular Weight:144.56

67-56-1
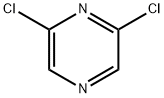
4774-14-5

33332-30-8
1. Methanol (MeOH, 0.27 mL, 6.7 mmol) was dissolved in 15 mL of tetrahydrofuran (THF). 2. Add sodium hydride (NaH, 60 wt% mineral oil dispersion, 0.32 g, 8.0 mmol) to the above solution and stir for 30 minutes at room temperature. 3. 2,6-dichloropyrazine (1.0 g, 6.7 mmol) dissolved in 20 mL of THF was added slowly and stirring was continued for 16 hours at room temperature. 4. Upon completion of the reaction, the reaction was quenched by the addition of aqueous ammonium chloride and the reaction mixture was extracted with ether (Et2O). 5. The organic layers were combined, dried over anhydrous magnesium sulfate, filtered and purified by column chromatography to afford the target product, 2-chloro-6-methoxypiperazine (0.71 g, 73% yield). 6. The structure of the product was confirmed by 1H-NMR (CDCl3): δ 8.14 (1H, s), 8.13 (1H, s), 3.99 (3H, s).

67-56-1
790 suppliers
$7.29/5ml-f

4774-14-5
356 suppliers
$5.00/1g

33332-30-8
109 suppliers
$7.00/100mg
Yield: 86%
Reaction Conditions:
Stage #1:methanol with sodium hydride in 1,2-dimethoxyethane at 0; for 0.333333 h;Inert atmosphere;
Stage #2:2,6-dichloropyrazine in 1,2-dimethoxyethane at 0 - 45;Inert atmosphere;
Steps:
2-(4-Methoxybenzyloxy)pyrazine (1c)
General procedure: To a suspension of NaH (0.645 g, 26.875 mmol) inanhydrous dimethoxyethane (30 mL) was slowly added dropwise 4-methoxybenzyl alcohol(3.34 mL, 26.907 mmol) at 0 °C over a period of 5 min and stirring continued for 15 min. Then,2-chloropyrazine (2 mL, 22.405 mmol) was added at 0 °C and the mixture heated at 45 °Covernight. The reaction mixture was quenched with slow addition of water (10 mL) andextracted with EtOAc (100 mL), washed with brine, dried over Na2SO4, concentrated underreduced pressure and the resulted crude syrup was purified by flash silica gel columnchromatography (Combiflash Rf) using EtOAc-hexanes (1:9) to obtain 1c (4.650 g, 99%) as awhite solid: mp: 69-71 °C; 1H NMR (CDCl3, 500 MHz) δ 8.25 (s, 1H), 8.11 (d, 1H, J = 3.0 Hz),8.08-8.10 (m, 1H), 7.39 (d, 2H, J = 8.5 Hz), 6.91 (d, 2H, J = 8.5 Hz), 5.32 (s, 2H), 3.81 (s, 3H);13C NMR (CDCl3, 125 MHz) δ 160.1, 159.6, 140.4, 136.6, 136.1, 130.0, 128.3, 113.9, 67.7,55.2; HRMS (ESI) m/z calcd for C12H12N2O2 [M+H]+ 217.09772, found 217.09715
References:
St Hilaire, Valentine R.;Hopkins, William E.;Miller, Yenteeo S.;Dandepally, Srinivasa R.;Williams, Alfred L. [Beilstein Journal of Organic Chemistry,2019,vol. 15,p. 72 - 78] Location in patent:supporting information

4774-14-5
356 suppliers
$5.00/1g

124-41-4
731 suppliers
$12.00/25g

33332-30-8
109 suppliers
$7.00/100mg
23902-69-4
1 suppliers
inquiry

33332-30-8
109 suppliers
$7.00/100mg
40155-28-0
138 suppliers
$20.00/1g

4774-14-5
356 suppliers
$5.00/1g

124-41-4
731 suppliers
$12.00/25g
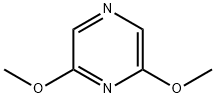
4774-15-6
143 suppliers
$7.00/250mg

33332-30-8
109 suppliers
$7.00/100mg

23902-69-4
1 suppliers
inquiry
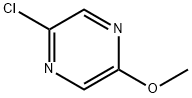
33332-31-9
86 suppliers
$42.00/100mg

33332-30-8
109 suppliers
$7.00/100mg

40155-28-0
138 suppliers
$20.00/1g