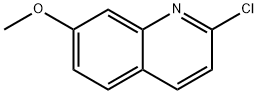
2-Chloro-7-Methoxyquinoline synthesis
- Product Name:2-Chloro-7-Methoxyquinoline
- CAS Number:49609-15-6
- Molecular formula:C10H8ClNO
- Molecular Weight:193.63
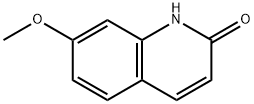
23981-26-2

49609-15-6
General procedure: 300 mg (1.9 mmol) of 7-methoxyquinolin-2(1H)-one was dissolved in 1.1 mL of phosphorus oxychloride (POCl3, 12 mmol) and the reaction was heated to reflux for 3 hours. Upon completion of the reaction, the reaction mixture was slowly poured into ice water, neutralized to neutral with saturated sodium bicarbonate (NaHCO3) solution and subsequently extracted with ethyl acetate. After treatment, the target product 2-chloro-7-methoxyquinoline was obtained in 98% yield. The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ 8.01 (d, J = 8.5 Hz, 1H), 7.68 (d, J = 8.9 Hz, 1H), 7.35 (d, J = 2.4 Hz, 1H), 7.25 (d, J = 8.3 Hz, 1H), 7.20 (dd, J = 8.9, 2.5 Hz, 1H), and 3.93 (s, 3H).

23981-26-2
32 suppliers
inquiry

49609-15-6
52 suppliers
inquiry
Yield:49609-15-6 98%
Reaction Conditions:
with trichlorophosphate for 3 h;Reflux;
Steps:
29.3 Step 3.2-Chloro-7-methoxyquinoline
300 mg (1.9 mmol) of 7-methoxyquinolin-2(1H)-one was dissolved in 1.1 mL of POCl3 (12 mmol) and heated at reflux for 3 h. The mixture was poured into iced water, neutralized with sat NaHCO3 and extracted with ethyl acetate. Yield 98%.1H NMR (400 MHz, CDCl3) δ 8.01 (d, J = 8.5 Hz, 1H), 7.68 (d, J = 8.9 Hz, 1H), 7.35 (d, J = 2.4 Hz, 1H), 7.25 (d, J = 8.3 Hz, 1H), 7.20 (dd, J = 8.9, 2.5 Hz, 1H), 3.93 (s, 3H).
References:
WO2016/130968,2016,A1 Location in patent:Page/Page column 183-184

23981-26-2
32 suppliers
inquiry

49609-15-6
52 suppliers
inquiry
536-90-3
298 suppliers
$10.00/5g

49609-15-6
52 suppliers
inquiry
15116-41-3
37 suppliers
inquiry

49609-15-6
52 suppliers
inquiry

