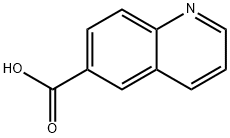
6-Quinolinecarboxylic acid synthesis
- Product Name:6-Quinolinecarboxylic acid
- CAS Number:10349-57-2
- Molecular formula:C10H7NO2
- Molecular Weight:173.17
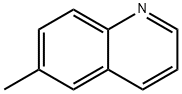
91-62-3

10349-57-2
6-Methylquinoline (100.0 mg, 0.70 mmol) was dissolved in a mixed solution of H2O (1.0 mL) and H2SO4 (0.25 mL) at 0 °C and chromium trioxide (272.0 mg, 2.72 mmol) was added in batches. The reaction mixture was refluxed for 24 hours. After completion of the reaction, it was cooled to room temperature and the precipitated crystalline bisulfate precipitate was removed by filtration. The filtrate was dissolved in 10% aqueous sodium hydroxide solution, washed with hexane and the pH was adjusted to acidic with acetic acid to precipitate 6-quinolinecarboxylic acid. The precipitate was collected to give 85.0 mg of 6-quinolinecarboxylic acid in 70% yield. The product could be used in subsequent steps without further purification. The product was characterized as follows: 1H NMR (300 MHz, DMSO-d6) δ 7.61 (dd, 1H, J1 = 8.3 Hz, J2 = 4.2 Hz), 8.08 (d, 1H, J = 8.8 Hz), 8.20 (dd, 1H, J1 = 8.8 Hz, J2 = 1.7 Hz), 8.56 (d, 1H, J = 8.2 Hz), 8.67 (m, 1H, J = 8.2 Hz), 8.67 (m, 1H, J = 8.2 Hz), 8.56 (d, 1H, J = 8.2 Hz), 8.56 (m, 1H, J = 8.2 Hz) 8.67 (m, 1H), 9.00 (dd, 1H, J1 = 4.1 Hz, J2 = 1.5 Hz), 13.20 (br s, 1H); 13C NMR (300 MHz, DMSO-d6) δ 122.9, 127.9, 129.2, 129.5, 130.0, 131.7, 138.2, 150.0, 153.4, 167.4, 167.0, 153.4, 167.4, 167.4, 167.4, 167.4, 167.4, 167.4 153.4, 167.7; ESI-MS m/z 196 [M + Na]+, 174 [M + H]+. Molecular formula: C10H7NO2.
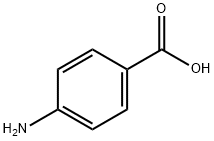
150-13-0
950 suppliers
$5.00/25g
56-81-5
1771 suppliers
$5.00/25g

10349-57-2
317 suppliers
$10.00/1g
Yield:10349-57-2 95%
Reaction Conditions:
with ferrous(II) sulfate heptahydrate;sulfuric acid;boric acid;4-nitro-benzoic acid at 140;
Steps:
73.1
Step 1. Quinoline-6-carboxylic acid Into a 500-mL 3-necked round-bottom flask, was placed 4-aminobenzoic acid (13.7 g, 100.00 mmol, 1.00 equiv), 4-nitrobenzoic acid (10.7 g, 64.07 mmol, 0.64 equiv), boric acid (5.9 g), FeSO4.7H2O (6.4 g), glycerol (38 mL), and concentrated sulfuric acid (18 mL). The resulting solution was stirred overnight at 140° C. The reaction mixture was cooled. The pH value of the solution was adjusted to 10 with sodium hydroxide (10%). The resulting solution was extracted with 2*100 mL of ethyl acetate and the aqueous layers were combined. The pH value of the aqueous layers was adjusted to pH 6 with conc. hydrochloric acid. The solids were collected by filtration. The resulting solution was diluted with 200 mL of methanol and the resulting solids were collected by filtration. The solid was dried to afford 17.3 g (95%) of quinoline-6-carboxylic acid as a dark-grey solid. LC-MS: (ES, m/z):174 [M+H]+
References:
BIOENERGENIX US2012/277224, 2012, A1 Location in patent:Page/Page column 82

91-62-3
274 suppliers
$11.92/5g

10349-57-2
317 suppliers
$10.00/1g
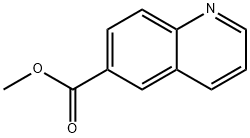
38896-30-9
176 suppliers
$10.00/1g

10349-57-2
317 suppliers
$10.00/1g
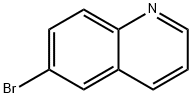
5332-25-2
400 suppliers
$6.00/5g

124-38-9
134 suppliers
$175.00/23402

10349-57-2
317 suppliers
$10.00/1g
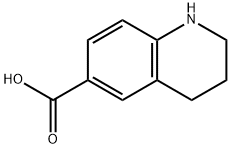
5382-49-0
89 suppliers
$32.00/100mg

10349-57-2
317 suppliers
$10.00/1g